US Pharm. 2012;37(3)(Oncology suppl):7-11.
ABSTRACT: The merging of nanotechnology and medicine has resulted in new approaches to designing specialized pharmaceutical formulations. While nanomedicine encompasses a diverse array of research fields, the focus of this review is on its therapeutic applications, specifically its impact on drug delivery and those products in clinical development. Within this focus, the field of oncology has felt the biggest impact from nanomedicine because of the exploitation of the enhanced permeability and retention effect in tumor tissues.
Because of the ubiquitous use of the prefix nano- and the term nanotechnology, defining nanomedicine is a challenging task. The European Science Foundation defines nanomedicine as “the science and technology of diagnosing, treating, and preventing disease and traumatic injury, of relieving pain, and of preserving and improving human health, using molecular tools and molecular knowledge of the human body.”1 However, the National Cancer Institute and the FDA follow a more restrictive definition: “the understanding and control of matter at dimensions between approximately 1 and 100 nanometers, where unique phenomena enable novel applications,” mirroring the definition of nanotechnology put forth by the National Nanotechnology Initiative.2,3 For most pharmaceutical applications, nanoparticles are defined as having a size up to 1,000 nm (i.e., 1 mcm).
No matter the disparities in defining the scope of the field, nanomedicine encompasses a broad range of nanotechnology research including both therapeutic and diagnostic applications. It is poised to revolutionize how medical and pharmaceutical industries approach the 21st century’s pharmaceutical care challenges.
Among its many emerging benefits, nanomedicine may facilitate the oral administration of drugs that are currently delivered only by injection. Nanoencapsulation of such drugs in a minuscule polymer or lipid matrix will allow them to easily pass through the gastrointestinal lining and reach the bloodstream where their payload will be released. Additionally, nanoparticles, which can be as small as a virus, can efficiently enter into diseased cells and facilitate more effective diagnosis and treatment. In whichever application, nanomedicine will drastically improve a patient’s quality of life by early detection and/or more efficient treatment with less drug-related side effects. Over all, expansion of these nanopharmaceuticals will improve the practice of medicine and clinical outcomes in the coming years.
The most notable nanomedicine advancements have been in the field of oncology. With the discovery of the enhanced permeation and retention (EPR) effect, passive targeting of chemotherapeutics to solid tumor tissues is an achievable objective given specific particle sizes and chemical characteristics.4 As its name implies, the EPR effect is the selective accumulation of macromolecules in solid tumor tissue and the retention of those macromolecules within the tissue for a prolonged time due to increased leakage of tumor blood vessels and decreased effective lymphatic drainage. FIGURE 1 summarizes the characteristics of tumor tissues, which permit the passage and retention of nanoparticles.
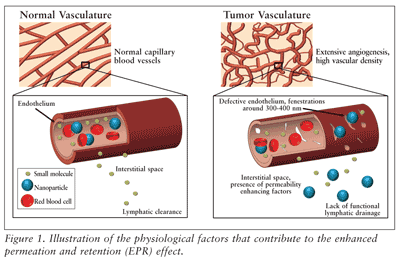
While small molecules can diffuse freely into normal tissues as well as tumor tissues, nanoparticles within a specific size range will not diffuse freely into normal tissues and will diffuse through the gaps in the endothelium of tumor tissues. Furthermore, the nanoparticles are retained within the tumor tissue for a prolonged time period because of the lack of adequate lymphatic clearance to remove the nanoparticles from the tumor. The size of the defects in the tumor vasculature is dependent on the cancer type, tumor site, and the stage of the disease, but the upper cutoff size is generally around 300 to 400 nm.5 Furthermore, therapeutics must be larger than 10 nm to avoid first-pass elimination in the kidney and smaller than 150 to 200 nm to avoid clearance in the liver and spleen.6 Thus, having nanoparticles with tunable size properties on the nanoscale (e.g., in the 20-100 nm range) is essential to taking advantage of the EPR effect and is one of the reasons that nanomedicine has become essential in the development and improvement of cancer therapeutics.
In addition to oncologic applications, nanomedicine research has stretched across the spectrum of medical specialties. Likewise, the array of nanotechnology products being applied to medicine is equally as varied: magnetic nanoparticles, quantum dots, liposomes, nanocrystals, nanosuspensions, gold nanoparticles, microspheres, carbon nanotubes, and other polymeric nanoparticle designs. While most of these technologies are still in preclinical development, there is a growing list of nanomedicine-enabled products already on the market and in clinical trials. This review focuses on those products and product candidates for therapeutic applications in medicine that are beyond preclinical development, discussing the purpose and advantages of integrating nanotechnology with pharmaceutics. TABLE 1 lists some of the FDA-approved drugs that have been developed or improved by nanomedicine techniques.7
The application of nanotechnology to the drug development process allows scientists to design and develop nanoscale pharmaceuticals that meet the size requirements necessary to achieve passive targeting. By virtue of their size and unique surface properties, nanoparticles are also capable of active targeting to diseased cells in order to deliver drugs at a higher concentration while reducing drug-related side effects by preventing or reducing the interaction with normal cells. Administration of drugs via nanoparticles allows manipulation of the absorption, distribution, metabolism, and elimination (ADME) of drugs and increases the overall therapeutic effect.
Lipid-Based Nanoparticles
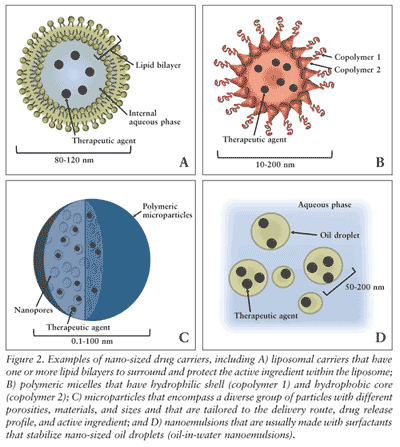
Liposomal drug carriers may be the most prolific nanomedicine technology currently on the market. Liposomes are composed of one or more concentric lipid bilayers encapsulating an inner aqueous core (FIGURE 2A). Their ubiquitous use in pharmaceutical formulations is because the outer lipid layer of liposomes protects the encapsulated drug from the external environment and because the outer surface can be functionalized to improve targeting. Some examples of liposomal drug formulations approved by the FDA are listed in TABLE 1.7
Indicated for recurrent ovarian cancer, relapsed/refractory multiple myeloma, and AIDS-related Kaposi’s Sarcoma, Doxil highlights some of the benefits of liposomal technology to improve drug delivery.8 Doxil’s liposomal shell surrounding the doxorubicin molecules is coated with polyethylene glycol (PEG). The liposomal carrier increases the blood circulation time of the drug, and the polymer PEG lends the liposome “stealth” qualities, largely avoiding the immune system. The prolonged blood circulation time of Doxil results in the passive accumulation of the drug at the tumor site by the EPR effect. Most importantly, the liposomal encapsulation limits the cardiotoxicity of doxorubicin.9
Numerous clinical trials confirmed the improved pharmacokinetics of doxorubicin when it is released from Doxil compared with the free drug. In a clinical trial as early as 1994, free doxorubicin and Doxil were compared in seven cancer patients after injection of equivalent doses of free doxorubicin and Doxil.10 Two dose levels were examined—25 and 50 mg/m2. Along with plasma levels, when possible, drug levels were also measured in malignant effusions. The plasma elimination of Doxil followed a biexponential pattern with half-life (t1/2) of 2 and 45 hours, respectively, and resulted in nearly a 300-fold increase in AUC when compared with free doxorubicin. In three patients, the pleural effusions/drug levels were 4- to 16-fold higher in Doxil-treated patients.10
In another study of 45 patients with breast cancer, the toxicity and pharmacokinetics of various doses of Doxil were evaluated as follows: 35 mg/m2 every 3 weeks, 45 mg/m2 every 3 weeks, 60 mg/m2 every 4 weeks, and 70 mg/m2 every 6 weeks.11 Doxil pharmacokinetics were described by a monoexponential elimination curve with a long t1/2 (79 h), a slow clearance (40 mL/h), and a small volume of distribution (3.9 L). Peak concentration (Cmax) and AUC increased linearly with the dose, with a statistically significant correlation. The toxicity of Doxil was dose and schedule dependent.11
Crucell’s virosome product, Inflexal V, utilizes 150-nm-diameter liposomes as the base of its vaccine formulation. The liposomes mimic the native virus structure, thus allowing cellular entry and membrane-fusion properties to the formulations.12 Liposomal nanotechnology also extends to cancer vaccines such as ImmTher, a liposome-encapsulated disaccharide tripeptide. ImmTher has been shown to have activity against liver and lung colorectal metastases in a phase I trial, and a phase II trial is ongoing assessing the 2-year disease-free survival of patients with high-risk Ewing’s sarcoma who are given vincristine, doxorubicin, cyclophosphamide, and dexrazoxane (VACdxr) with and without ImmTher.13,14 All of the FDA-approved liposomal drug formulations and those still in the development pipeline represent a wide range of delivery routes, indications, and compounds, which attests to the flexibility and versatility of liposomal formulations.
Polymer-Based Nanoparticles
Polymeric nanoparticles provide the solution to some of the most persistent challenges in drug delivery: drug solubility and stability, circulation half-life, and reduction of toxicity to non-target tissues. Polymeric nanoparticles are defined by their structure and polymer composition, with the therapeutic agent conjugated to the surface or interior of the nanoparticle.6 Some of the general advantages of polymeric nanoparticles are the ability to alter the release profile of the drug, the ability to control the targeting of the therapeutic through active or passive targeting, and the ability to minimize its degradation within the bloodstream.15
Polymeric nanoparticle products approved by the FDA are listed in TABLE 1.7 From this list, nine of the drugs incorporate PEGylation technology, the covalent attachment of the polymer PEG to the drug or to the drug carrier.
By controlling the number of PEG chains, the molecular weight and the structure of the PEG chains, and the attachment chemistry, PEGylation can shield active ingredients from recognition and degradation by the immune system, can reduce renal filtration, and can alter the biodistribution of the drug by increasing the circulation half-life.16 An increase in the circulation half-life reduces the overall dosage of the drug by reducing the frequency of its administration. PEGylation technology has most impacted the delivery of therapeutic proteins, such as enzymes, hormones, and antibodies, which are naturally unstable and have very short circulating half-lives. The drug candidate Aurimune (CYT-6091) conjugates PEG to the surface of 27 nanometer-size colloidal gold particles to avoid immune detection within the bloodstream, thereby allowing its active ingredient, recombinant human tumor necrosis factor-alpha (TNF-alpha), to reach the tumor tissues. Phase I trials for Aurimune demonstrated that considerably higher doses of TNF are possible with Aurimune’s carrier system, and accumulation of the drug was observed in and around tumor sites.17
Polymeric Micelles
Similar to the spheroidal structure of liposomes, micelles are aggregates of surfactant or polymer dispersed in an aqueous solution but do not have an internal aqueous phase like that of liposomes (FIGURE 2B). Polymeric micelles have the same advantages as other polymeric formulations and liposomal formulations: namely, the protection of the therapeutic agent from degradation and increased circulation time of the drug. Generally, polymeric micelles are made with two different polymers: a hydrophilic shell that is responsible for colloidal stability and protects the active ingredient, and a hydrophobic core polymer that either physically and/or chemically protects the active ingredient.18 As seen in other platform technologies, polymeric micelle technology has advanced furthest in the field of oncology.
Genexol-PM, developed by the South Korean company Samyang Corporation, is a PEG-poly (lactic acid) micelle formulation of paclitaxel. These micelles are 20 to 50 nm in size. The two main objectives of Genexol-PM are to reduce Cremophor EL-related toxicities and to increase therapeutic efficacy.19 Cremophor EL is a synthetic surfactant excipient used to dissolve paclitaxel in Taxol (paclitaxel, Bristol-Myers Squibb) and requires premedication to negate its side effects. Encasing the paclitaxel within the micelle negates the use of Cremophor EL by protecting the paclitaxel molecules within the hydrophobic core of the micelle and by maintaining high aqueous solubility because of the micelle’s hydrophilic PEG shell.
A single-arm, multicenter phase II clinical trial was conducted to evaluate the safety and efficacy of Genexol-PM in metastatic breast cancer patients.19 Forty-one patients received Genexol-PM 300 mg/m2 by IV infusion over 3 hours every 3 weeks without premedication. Overall response rate was 58.5% with 5 complete responses and 19 partial responses. The median time to progression for all patients was 9.0 months. No febrile neutropenia was observed in any of the participants. Currently, Genexol-PM has ongoing phase II trials for pancreatic cancer, ovarian cancer, and non-small-cell lung cancer, and phase III and phase IV trials in patients with recurrent breast cancer, studying the toxicity, progression-free survival, and tumor control rate.20
Microparticles and Nanoparticles
Microspheres and microcapsules, collectively referred to as microparticles, have been widely used in pharmacy for drug delivery. These drug delivery systems contain a variety of polymers, including both biodegradable and nonbiodegradable. Microparticles are used in drug formulations to control the release of the drug, to protect sensitive therapeutics from degradation, and to allow for surface functionality of the microparticle for targeting and delivery (FIGURE 2C). The degradation rate and drug release rate of microparticles are controlled by the material choice, porosity, surface properties, and the size of the microparticles. Admittedly larger than traditional nanomedicine products, microparticles are usually on the scale of microns rather than nanometers but have similar properties and functions as those particles that fit within the rigid definition of nanotechnology.
Yet another paclitaxel formulation, AI-850, is a polymeric formulation utilizing sponge-like sugar microspheres (<2 mcm diameter) to increase the solubility of the paclitaxel nanoparticles, to reduce the IV infusion time, and to eliminate the need for premedication with the elimination of Cremophor EL. While the phase I studies did not show evidence that AI-850 is superior to published data on other paclitaxel formulations, the study did show that the microsphere delivery system could be a potential alternative for paclitaxel delivery and warrants further research, especially since the infusion time was reduced in this study and no premedication was administered.21 Developed by Acusphere, Inc., the worldwide rights to AI-850 were licensed to Cephalon in 2008.
Another oncological candidate comes from Flamel Technologies, Inc. IL-2 XL is a complex of interleukin-2 (IL-2) with Flamel’s Medusa controlled-release delivery system that is targeted at renal cell carcinoma. Their Medusa nanoparticle delivery system contains a polymer that spontaneously forms a stable nanogel in water. The nanogel is made of 20 to 50 nm nanoparticles containing the captured active ingredient for the extended release of the protein or peptide.22 Some advantages of the Medusa system are the reduction of burst release of the drug, the ability to incorporate a wide range of proteins and peptides, and the system’s biocompatibility and biodegradable properties. The phase I/II trial for IL-2 XL demonstrated that Flamel’s formulation could compete with the approved IL-2 treatments and has the potential for better efficacy through an increased immunologic cellular response and sustained pharmacodynamic response.22
Protein-Based Nanoparticles
Similar to the polymeric nanoparticles, natural proteins are also used to improve the pharmacokinetics and toxicity of current drug formulations. Abraxane, paclitaxel protein-bound particles of approximately 130 nanometers, was first approved by the FDA in 2005 for metastatic breast cancer. By stabilizing the paclitaxel particles with serum albumin, no solvent, such as Cremophor-EL, is needed in the formulations, thereby improving the infusion time and eliminating the need for premedications.23
In a phase III clinical trial, 454 breast cancer patients were randomly assigned to 3-week cycles of either albumin-bound paclitaxel (Abraxane) 260 mg/m2 intravenously without premedication (n = 229) or standard paclitaxel 175 mg/m2 intravenously with premedication (n = 225). Abraxane demonstrated significantly higher response rates (33%) compared with standard paclitaxel (19%). Abraxane treatment also provided significantly longer time to tumor progression (23.0 weeks) compared with standard paclitaxel (16.9 weeks). The incidence of grade 4 neutropenia was significantly lower (9%) for Abraxane compared with standard paclitaxel (22%).24
Two further albumin-bound drug formulations utilizing the same technology as Abraxane, ABI-008 (nab-docetaxel) and ABI-009 (nab-rapamycin) from Abraxis BioSciences, which is now a subsidiary of Celgene Corporation, are currently in active clinical trials. Abraxis’ nab technology stands for nanoparticle albumin-bound, whose nanoparticles exploit the natural carrier properties of albumin as well as taking advantage of the EPR effect. ABI-008 is in phase I/II clinical trials for hormone-refractory prostate cancer, and ABI-009 is in phase I trials for patients with advanced nonhematologic malignancies.25
Additional Nanomedicine Platforms
Another field of research under the umbrella of nanomedicine is the utilization of nanoemulsions in drug delivery (FIGURE 2D). Nanoemulsions are stabilized nano-sized oil droplets emulsified in water. The oil droplets can range in size from 10 to 500 nm in diameter and act as carriers for water-insoluble drug compounds. NanoBio Corporation’s NB-001 is a nanoemulsion formulation, consisting of 180-nm oil droplets emulsified in water.26 NB-001 is indicated for herpes labialis infection and has demonstrated its safety, efficacy, and tolerability in phase II trials involving over 800 patients. The nano-sized oil droplets purportedly cross the skin through pores and hair follicles and accumulate in the epidermis and dermis, directly acting at the site of infection. In 2009, NanoBio and GlaxoSmithKline (GSK) announced an exclusive licensing agreement for the OTC use of NB-001, building on GSK’s Abreva brand.27
Nano-Cancer therapy, from MagForce Nanotechnologies AB, is yet another nanomedicine therapy for cancer but is distinctive in its mode of action. Coated iron oxide nanoparticles approximately 20 nm in diameter (NanoTherm therapy) are locally delivered to tumor tissues, followed by the application of a magnetic field to cause the nanoparticles to vibrate. The vibration of the iron oxide nanoparticles generates heat, killing the surrounding tumor cells. Both phase I and phase II trials have been completed in various tumor types, including glioblastoma multiforme and prostate, cervical, esophageal, pancreatic, and breast cancers.28,29
Future of Nanomedicine
The impact of nanomedicine in drug delivery is unmistakable. The product candidates discussed in this review are not the only nanomedicine products being investigated in clinical trials today, but they do accurately represent the spectrum of nanoparticles used in drug delivery. Moreover, this review does not take into account the thousands of other products that are in preclinical development. With adequate evaluation of the possible toxicities of nanoparticles and with continued nanotechnology innovations related to complex diseases such as cancers, the list of nanomedicine-enabled products will continue to experience positive growth in the future.
REFERENCES
1. European Science Foundation. ESF Forward Look on Nanomedicine. Strasbourg, France: European Science Foundation; 2005.
2. National Nanotechnology Initiative. Strategic Plan,
December 2007. Washington, DC: National Science and Technology Council;
2007.
3. National Cancer Institute. Cancer Nanotechnology
Plan: A Strategic Initiative To Transform Clinical Oncology and Basic
Research Through the Directed Application of Nanotechnology. Washington, DC: U.S. Department of Health and Human Services; July 2004.
4. Matsumura Y, Maeda H. A new concept for macromolecular
therapeutics in cancer chemotherapy: mechanism of tumoritropic
accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-6392.
5. Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:99-112.
6. Alexis F, Pridgen E, Molnar LK, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505-515.
7. FDA approved drug products. Drugs@FDA. www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed February 8, 2011.
8. Duggan ST, Keating GM. Pegylated liposomal doxorubicin:
a review of its use in metastatic breast cancer, ovarian cancer,
multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 2011;71:2531-2558.
9. Plosker GL. Pegylated liposomal doxorubicin: a review
of its use in the treatment of relapsed or refractory multiple myeloma. Drugs. 2008;68:2535-2551.
10. Gabizon A, Catane R, Uziely B, et al. Prolonged
circulation time and enhanced accumulation in malignant exudates of
doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987-992.
11. Lyass O, Uziely B, Ben-Yosef R, et al. Correlation of
toxicity with pharmacokinetics of pegylated liposomal doxorubicin
(Doxil) in metastatic breast carcinoma. Cancer. 2000;89:1037-1047.
12. Herzog C, Hartmann K, Künzi V, et al. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381-4387.
13. Vosika GJ, Cornelius DA, Gilbert CW, et al. Phase I
trial of ImmTher, a new liposome-incorporated lipophilic disaccharide
tripeptide. J Immunother (1991). 1991;10:256-266.
14. Vincristine, doxorubicin, cyclophosphamide and
dexrazoxane (VACdxr) in high risk Ewing’s sarcoma patients.
http://clinicaltrials.gov/show/NCT00038142. Accessed February 8, 2012.
15. Brewer M, Zhang T, Dong W, et al. Future approaches of nanomedicine in clinical science. Med Clin North Am. 2007;91:963-1016.
16. Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54:459-476.
17. Libutti SK, Paciotti GF, Myer L, et al. Preliminary
results of a phase I clinical trial of CYT-6091: a pegylated colloidal
gold-TNF nanomedicine. J Clin Oncol (ASCO Annual Meeting). 2007;25(18S):A3603.
18. Talelli M, Rijcken CJ, van Nostrum CF, et al. Micelles based on HPMA copolymers. Adv Drug Deliv Rev. 2010;62:231-239.
19. Lee KS, Chung HC, Im SA, et al. Multicenter phase II
trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of
paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108:241-250.
20. Genexol-PM (paclitaxel). www.clinicaltrials.gov. Accessed February 8, 2012.
21. Mita AC, Olszanski AJ, Walovitch RC, et al. Phase I
and pharmacokinetic study of AI-850, a novel microparticle hydrophobic
drug delivery system for paclitaxel. Clin Cancer Res. 2007;13:3293-3301.
22. Chan YP, Meyrueix R, Kravtzoff R, et al. Review on
Medusa: a polymer-based sustained release technology for protein and
peptide drugs. Expert Opin Drug Deliv. 2007;4:441-451.
23. Desai N, Trieu V, Yao Z, et al. Increased antitumor
activity, intratumor paclitaxel concentrations, and endothelial cell
transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared
with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317-1324.
24. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase
III trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel
in women with breast cancer. J Clin Oncol. 2005;23:7794-7803.
25. Vishnu P, Roy V. Nab-paclitaxel: a novel formulation of taxane for treatment of breast cancer. Womens Health. 2010;6:495-506.
26. Pannu J, McCarthy A, Martin A, et al. NB-002, a novel
nanoemulsion with broad antifungal activity against dermatophytes, other
filamentous fungi, and Candida albicans. Antimicrob Agents Chemother. 2009;53:3273-3279.
27. NanoBio, with Glaxo as big partner, sees market in
treating and (maybe) preventing cold sores. NanoBio Corporation. July
15, 2010. www.nanobio.com/News/Press-Releases.html. Accessed February 8,
2012.
28. Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy
and safety of intratumoral thermotherapy using magnetic iron-oxide
nanoparticles combined with external beam radiotherapy on patients with
recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317-324.
29. Johannsen M, Gneveckow U, Thiesen B, et al.
Thermotherapy of prostate cancer using magnetic nanoparticles:
feasibility, imaging, and three-dimensional temperature distribution. Eur Urol. 2007;52:1653-1661.
To comment on this article, contact rdavidson@uspharmacist.com.





