US Pharm. 2012;37(4)(Compliance suppl):8-11.
Approximately half of the U.S. population uses at least one prescription drug. During hospital outpatient department visits alone, 248 million drugs are ordered annually, and 75% of visits involve drug therapy.1 While these medications have substantial therapeutic benefits for their users, they can also confer serious risks leading to injury, hospitalization, or death. An estimated 700,000 Americans are treated annually for adverse drug events (ADEs) in emergency departments, with about 117,000 patients being admitted.1 Regular laboratory monitoring is a key tool in the detection and prevention of these toxicities. Additionally, it detects suboptimal drug levels and guides clinical decisions. Routine lab monitoring is especially important, as both suboptimal and toxic levels may manifest asymptomatically while having the potential for significant morbidity and mortality.
Background
The elderly population is particularly susceptible to the dangers of insufficient lab monitoring. Among these patients, approximately 85% of emergency department visits due to ADEs and 87% of overdoses requiring hospitalization involve outpatient medications that require routine lab monitoring.1 In a study evaluating the incidence and preventability of ADEs among elderly patients in the ambulatory setting, the majority of errors associated with preventable events occurred because of inadequate monitoring or failure to respond to abnormal lab findings.2 As lifespans and medication usage continue to rise, these percentages are bound to increase.
Although monitoring is essential for guiding therapy and preventing ADEs, compliance among patients remains low and inconsistent. In one study, 50% of patients receiving medications with a narrow therapeutic index were not monitored during a 1-year period.3 Another study reported that up to 39% of patients had outdated lab tests.4 Reasons for low rates of compliance with monitoring include lack of education, funds, and accessibility. In addition, monitoring guidelines may recommend relatively infrequent laboratory tests, and time lapses can make it difficult for patient and clinician alike to remember when a lab is due.
Reminder Interventions
Because many ADEs and suboptimal drug levels are preventable with lab monitoring, a reminder process that alerts patients to obtain necessary tests can prevent drug-related complications. Several studies have analyzed the utilization of reminder interventions, with mixed results. Hoch and colleagues found that onscreen reminders on computerized medical records led to a 9.8% relative increase in clinician-prompted potassium testing in patients receiving diuretics.5 However, other studies noted a tendency for clinicians to bypass computerized alerts.6
While previous studies evaluated the effect of clinician reminders, other studies have used a patient-directed approach. In a study by Raebel and colleagues, a pharmacist contacted patients by phone if baseline tests were incomplete, resulting in a significant improvement in compliance rates.7 A review of studies evaluating the effectiveness of automated telephone reminders showed an overall increase in patient adherence to medications and appointments.8
Feldstein and colleagues compared the effectiveness of three reminder interventions: an electronic medical-record reminder sent to the primary care physician, an automated voice message sent to the patient, and a pharmacy-driven phone call followed by a letter.9 The pharmacy-driven intervention resulted in higher compliance rates compared with the automated voice message. The computer alert to the physician was least effective; additionally, the physicians preferred the patient-directed approaches. This study not only demonstrated that direct patient interaction led to better outcomes than clinician-directed interventions, but also advocated the use of pharmacy-led interventions for decreasing medication errors and ADEs while reducing the burden on outpatient physicians. As gatekeepers of medication therapy, pharmacists are in a unique position to detect outdated labs and to notify providers and patients accordingly.
Pharmacist-Driven Reminder Process
In January 2008, a pharmacist-driven reminder process involving a customized comment (sig comment) added to prescription-bottle label instructions (FIGURE 1) was instituted at Jesse Brown VA Medical Center (JBVAMC). In March 2010, a reminder letter was also implemented. Many of these reminders came with a suggested lab-completion date. This was done in an effort to coordinate the lab draw with a scheduled clinic appointment. Targeted for these interventions were patients taking medications requiring routine monitoring who did not have updated labs within the last year or sooner, if needed. Patients who had not completed labs ordered by a clinician and those who were starting new medications requiring frequent monitoring also received a reminder intervention. Requests were based on package inserts and clinical guidelines. Requests for lab testing were placed by either the physician or a staff pharmacist under the physician’s name through a collaborative agreement. The physician received the lab results, including any critical lab values.
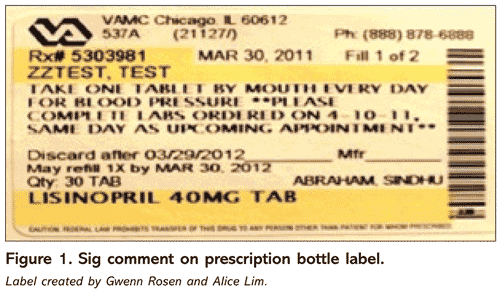
Although studies have identified reminder methods that improve lab-test compliance, literature specifically assessing the use of a customized comment on the prescription label and/or reminder letter is limited. This study evaluated the effectiveness of the two reminder interventions used at JBVAMC and assessed the impact on medication safety and efficacy by examining follow-up clinical interventions conducted as a result of these reminders.
Methodology
This study, performed from January 1, 2008, to September 15, 2010, was an Institutional Review Board and VA Research and Development Committee–approved retrospective electronic chart review. A list of patients who received a sig comment was generated from the computerized patient-record system. Another list was generated using an outpatient pharmacy log, which documented patients who received a reminder letter. If patients appeared on both lists, it was indicated that they received both interventions.
Patients were divided into three groups: sig comment only, letter only, and sig comment plus letter. Patients who appeared on one or both lists during the specified study period and who received reminders for target medications were included. Target medications were chosen because of their prevalent usage at JBVAMC and also because past medication-use evaluations at the facility identified them as problematic drugs to monitor (TABLE 1).10-22 Patients who received additional interventions (phone calls, voice messages), those hospitalized during the 90-day follow-up period before completing requested labs, those with home-based primary care (HBPC), those with labs ordered after the follow-up period, and those whose labs were completed before the reminder intervention occurred were excluded.
The primary outcome was overall lab compliance rate after any reminder intervention. Compliance rates of the three patient groups also were compared. For the purposes of this study, compliance was defined as the completion of requested labs within 90 days of the intervention date. Secondary endpoints included an evaluation of suboptimal lab findings as related to the safety and efficacy of the medication. Clinical interventions made as a result of suboptimal findings were analyzed. The chi-square test was used to evaluate primary endpoints, and the Wilcoxon rank-sum and Kruskal-Wallis tests were used to assess patient characteristics. An alpha level of .05 was considered statistically significant.
Results
Of 841 patients identified from the computerized record system as receiving a sig comment, 85 were randomized into the sig comment–only group (15 of these were excluded). A total of 25 patients received a letter only, and six were excluded. A total of 70 patients received both interventions; of these, 17 were excluded. This yielded a total enrollment of 142 patients. Of the baseline characteristics assessed, the average number of comorbidities was significantly higher in the letter-only group compared with the other groups. Also, the average number of appointments in the previous year was significantly lower in the sig comment–only group than in the other groups. Baseline characteristics were otherwise balanced.
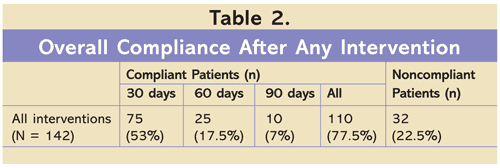
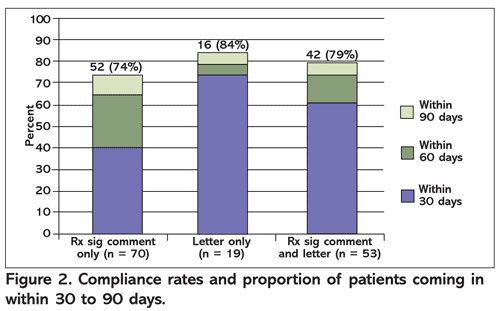
For the primary endpoint, 110 patients (77.5%) were compliant with requested lab tests (TABLE 2). A majority of patients (n = 75, 53%) completed labs within 30 days. The letter-only intervention led to the highest rate of compliance (84%) and to the largest proportion of patients who came in within 30 days (FIGURE 2). This was followed by the sig comment and letter combination group (79%) and the sig comment–only group (74%). There was no statistically significant difference between interventions, however.
When the characteristics of compliant and noncompliant patients were compared, only the number of appointments in the previous year was statistically different. Compliant patients had an average of 14 appointments in the last year, compared with seven appointments for noncompliant patients (P = .048). Other factors, including presence of a suggested lab date, did not appear to influence compliance rates.
Ordered lab tests that were associated with the target medications were referred to as target labs. In many patients, other lab tests were ordered that were not associated with the target medications; these were termed nontarget labs. Of patients who complied with requested tests, 49% had at least one suboptimal lab finding; 18 patients had a suboptimal target lab; 27 had a suboptimal nontarget lab; and nine had both suboptimal target and nontarget labs. In 45 patients, an intervention was made in response to a suboptimal lab finding. The most common intervention was starting a new medication.
Three patients visited the emergency department or were hospitalized as a result of a suboptimal lab. The first patient had a hemoglobin of 6.5 g/dL and a hematocrit of 23.8%. She returned to the emergency department for a transfusion and was started on iron supplementation and ranitidine, meloxicam was discontinued, and a colonoscopy was scheduled. Another patient had a potassium level of 6.0 mmol/L. He returned to the emergency department for a repeat level and cardiac monitoring. The repeat level was 4.9 mmol/L, the ECG was normal, and the patient was sent home without being admitted. A third patient, who had B-cell lymphoproliferative disorder, was found to have a WBC count of 60.8 K/mcl, a uric acid level of 10.0 mg/dL, and a creatinine level of 2.1 mg/dL. He was admitted for tumor lysis syndrome and was given IV fluids until serum creatinine decreased to his baseline of 1.4 mg/dL and uric acid declined to 6.3 mg/dL. He was discharged 2 days later with prescriptions for allopurinol, prednisone, and calcium and vitamin D supplementation.
No change was made in nine patients with suboptimal labs. In three of these patients, the clinician acknowledged the finding, but made no changes. In another three, the clinician had started a target medication before baseline labs were completed, so the patient received a reminder intervention. Therefore, when the suboptimal lab result returned, no change was made because the patient already had started the target medication.
Discussion
After receiving any pharmacy-driven reminder intervention, 77.5% of patients were compliant with requested lab monitoring within 90 days of receiving the intervention. A majority of patients completed the lab within 30 days, a finding that further strengthens the conclusion that increased compliance was directly related to the reminder interventions. There was no difference in compliance rates between the three intervention groups. The only factor that appeared to influence compliance rates was average number of completed appointments in the previous year. Otherwise, the characteristics of patients who were compliant and those who were noncompliant were similar. This finding supports the applicability of these interventions to a wide range of patient populations.
These reminder interventions were effective in increasing compliance and also led to many necessary clinical interventions, including three visits to the emergency department, one of which resulted in a hospitalization. Many suboptimal labs were nontarget or incidental lab findings, demonstrating that not only are these reminder interventions important in the regular maintenance of target medications, but they also bring attention to other labs that might otherwise be left unchecked.
This study had several limitations. The study was retrospective, baseline characteristics between intervention groups were unbalanced, and the lack of documentation and outside medical records may have skewed results. The small patient population and the specific veteran population studied may weaken the generalizability of these results. Additionally, this study did not assess other factors that may influence compliance rates, such as cost and distance of travel to the lab.
Conclusion
Despite the importance of laboratory monitoring, compliance with lab monitoring guidelines remains poor among patients. Pharmacists can help prevent ADEs and hospitalizations due to a lack of routine lab monitoring by simply reminding patients to complete labs. This study demonstrates that a customized comment added under the dosing instructions on a prescription bottle label and/or reminder letter are effective ways to improve lab compliance. The study also supports an emerging role for outpatient dispensing pharmacists in which they can further impact patient outcomes while minimizing the burden on physicians in the ambulatory setting. Although the results of this study are promising, larger prospective studies with more standardized methods of reminding patients are needed in order to fully evaluate the efficacy and benefit of these interventions.
REFERENCES
1. Budnitz DS, Pollock DA, Weidenbach KN, et al. National
surveillance of emergency department visits for outpatient adverse drug
events. JAMA. 2006;296:1858-1866.
2. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and
preventability of adverse drug events among older persons in the
ambulatory setting. JAMA. 2003;289:1107-1116.
3. Raebel MA, Carroll NM, Andrade SE, et al. Monitoring of drugs with a narrow therapeutic range in ambulatory care. Am J Manag Care. 2006;12:268-274.
4. Smith DH, Feldstein AC, Perrin NA, et al. Improving laboratory
monitoring of medications: an economic analysis alongside a clinical
trial. Am J Manag Care. 2009;15:281-289.
5. Hoch I, Heymann AD, Kurman I, et al. Countrywide computer alerts
to community physicians improve potassium testing in patients receiving
diuretics. J Am Med Inform Assoc. 2003;10:541-546.
6. Lo HG, Matheny ME, Seger DL, et al. Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc. 2009;16:66-71.
7. Raebel MA, Lyons EE, Andrade SE, et al. Laboratory monitoring of drugs at initiation of therapy in ambulatory care. J Gen Intern Med. 2005;20:1120-1126.
8. Krishna S, Balas EA, Boren SA, Maglaveras N. Patient acceptance of
educational voice messages: a review of controlled clinical studies. Methods Inf Med. 2002;41:360-369.
9. Feldstein AC, Smith DH, Perrin N, et al. Improved therapeutic monitoring with several interventions: a randomized trial. Arch Intern Med. 2006;166:1848-1854.
10. Allopurinol product information. Corona, CA: Watson Pharmaceuticals, Inc; January 2006.
11. Colcrys (colchicine) product information. Philadelphia, PA: AR Scientific, Inc; July 2011.
12. Prinivil (lisinopril) product information. Whitehouse Station, NJ: Merck & Co, Inc; November 2011.
13. Plavix (clopidogrel) product information. Bridgewater, NJ:
Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; December 2011.
14. CellCept (mycophenolate mofetil) product information. South San Francisco, CA: Genentech USA, Inc; February 2010.
15. Prograf (tacrolimus) product information. Deerfield, IL: Astellas Pharma US, Inc; February 2012.
16. Glucophage (metformin) product information. Princeton, NJ: Bristol-Myers Squibb; January 2009.
17. Hydrochlorothiazide product information. Morristown, NJ: Watson Pharma, Inc; February 2011.
18. Lasix (furosemide) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; August 2011.
19. Crestor (rosuvastatin) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; February 2012.
20. Zocor (simvastatin) product information. Whitehouse Station, NJ: Merck & Co, Inc; February 2012.
21. Renagel (sevelamer) product information. Cambridge, MA: Genzyme Corp; November 2007.
22. Rheumatrex (methotrexate) product information. Fort Lee, NJ: DAVA Pharmaceuticals, Inc; July 2009.
To comment on this article, contact rdavidson@uspharmacist.com.






