US Pharm. 2012;37(6)(Generic Drug Review):8-10.
According to the FDA, a majority of the prescriptions dispensed in the United States are for generic drugs. The use of generics in lieu of brand-name products is expected to grow, since the patent protection of a sizable number of brand-name drugs will expire by 2015. According to testimony before the U.S. House of Representatives concerning generic drug user fees, more than two-thirds of all prescriptions dispensed in the U.S. are for generic drugs. In 2011, approximately 78% of the more than 3 billion new and refilled prescriptions dispensed were filled with generics. In the last decade, generic drugs have saved the nation’s health care system more than $931 billion, as stated in the testimony.
Generic Drug User Fee
The FDA plays a pivotal role in making it possible for consumers to quickly avail themselves of the generic versions of brand-name drugs. Under the proposed user fee program, the FDA would charge pharmaceutical manufacturers a fee to help fund a portion of the agency’s drug review activities, while in return the FDA would aim to shorten its review time for generic drugs. Currently, 1,000 complete Abbreviated New Drug Applications are electronically submitted annually. The projected $299 million revenue from generic drug user fees over a 5-year period is expected to enable the FDA to act on 90% of complete electronic Abbreviated New Drug Applications within 10 months—as opposed to the current median time of 31 months—by significantly increasing the FDA’s productivity without compromising the agency’s high standards. The proposed user fee programs for generic drugs and biosimilars are based on the successful Prescription Drug User Fee Act program, which over the past 20 years has helped shorten the time it takes to get medications into patients’ hands.
Bioequivalence
A generic drug is mandated by the FDA to have the same active ingredient, strength, dosage form, and route of administration as the brand-name product it is bioequivalent to. Inactive ingredients in the generic drug formulation are permitted to differ from those contained in the brand-name drug. The rigorous standards established by the FDA ensure that a generic product released to market works equally as well as the brand-name drug. Through a review of bioequivalence data, the FDA determines whether the performance of a generic product is the same as that of its brand-name counterpart. A generic drug must establish that it is identical in all respects to the brand-name drug before it is permitted by the FDA to be released into the distribution channel. The FDA mandates that the difference in the generic-to-brand comparison be about the same as that for the brand-to-brand comparison; the exception is a generic drug modeled after a single brand-name drug, which must perform approximately the same in the body as the brand-name drug. There will always be a slight—but medically insignificant—level of natural variability, just as there is between different batches of a brand-name product.
Price Advantage of Generics
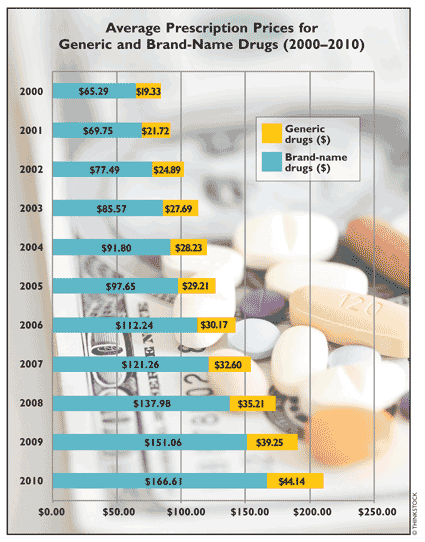
The rate of growth of generic prescriptions in the U.S. is attributed primarily to the fact that generic products are a cost-effective way to cure or mitigate the health problems of the American public. There is a big price differential between generic and brand-name drugs. Between 2000 and 2010, as shown in the graph, the average cost of a generic drug was 68% to 75% lower than that of the brand-name product, yielding an average of 71.1% per annum across the decade. The price of the average generic prescription dispensed increased from $19.33 in 2000 to $44.14 in 2010, for an overall 128% increase in price across the decade. However, the average annual rate of increase in the cost of generic and brand-name prescriptions did not show much of a difference—i.e., 9.53% for generic drugs in contrast to 9.86% for brand-name drugs. In 2004 and 2007, the rate of increase of the average generic prescription price was in the single digits—i.e., between 2% and 8%, while brand-name drug prescriptions had the lowest rate of price increase—6.4%—between 2004 and 2005.
Drug Shortages and Generics
The FDA tracked 127 drug shortages from January 2010 to October 2011. Fifty percent of these drugs were generics and 43% were innovator drugs; 80% of the 127 drugs were sterile injectable medications. In 2010, the top five manufacturers of generic sterile injectables produced 80% of these products sold in the U.S. No single cause accounts for shortages of drugs. Fifty-four percent of drug shortages were due to quality issues such as particulates, microbial contamination, impurities, and stability changes.
To comment on this article, contact rdavidson@uspharmacist.com.






