US Pharm. 2012;37(6)(Generic suppl):30-38.
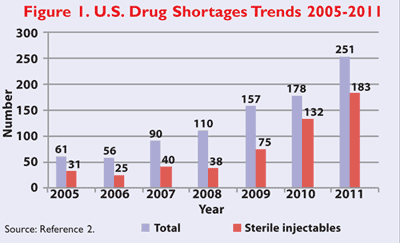
The United States is facing a growing crisis of drug shortages that is delaying and endangering patient care. Though a small minority of all drugs marketed in the U.S. experience a shortage in any given year, the number of shortages has been increasing annually since 2006. While injectable drugs are a small percentage of the overall drug market, they represent a disproportionate share of the shortages of the most critically needed drugs such as chemotherapy agents, antibiotics, morphine, and electrolyte/nutritional products. This disproportionate shortage of generic injectable medications is stressing the health system not only with compromised patient care, but also with escalating costs for the very limited supplies of the critical drugs that are available. At least 15 deaths since 2010 have been linked to these shortages.1 The pattern of shortages in the past was equal for brand and generic, but the trend recently has been mainly in the generic industry. Over 251 shortages occurred in 2011 across all categories of medications; however, roughly 75% (183) of these shortages continue to be sterile injectable drugs (FIGURE 1).2
Why Are Drug Shortages Becoming More Common?
The stated reasons for drug shortages range from reduced availability of raw materials to regulatory issues and manufacturing problems; however, one of the more likely causes of these shortages is the issue of shrinking profit margins. Manufacturers’ profits depend on their ability to develop and implement effective business strategies. Strategies for dealing with low profit lines include minimizing backup supplies by lowering inventories and, at times, discontinuing unprofitable product lines. On February 21, 2012, the FDA announced that it would temporarily allow importation of a replacement drug for Doxil, a drug for cancer treatment that has not been available for patients in need of treatment. In this same press release, FDA Commissioner Margaret A. Hamburg, MD, told the Associated Press that “generic injected drugs are the workhorses for hospitals but are difficult to make and produce little profit for drug makers.”1
The rapidly escalating percentage of injectable product shortages has been further complicated by the increased attention to the quality control requirements for these sterile products. The FDA has heightened efforts to enforce “good manufacturing practices,” which may result in production gaps while the manufacturer addresses these deficiencies.2 One of the suggested interventions has been to encourage other manufacturers to produce the injectable products during shortages. The manufacturing process for injectable medications is, however, very complicated, and alternative manufacturers may not be able or willing to assist in production. Additionally, the limited resources within the FDA have stifled the agency’s ability to return to problematic facilities in order to give the green light to resume production in a timely manner.
The definition of shortage can also vary, which can result in different interpretations of the crisis. The American Society of Health-System Pharmacists (ASHP) defines a shortage as a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.2 The FDA, on the other hand, defines a shortage with a focus on the product used to prevent or treat a serious or life-threatening disease or medical condition for which there is no other available source with sufficient supply of that product or alternative drug available.2
The economics of the shortage epidemic has been surprising to many, as prices generally fluctuate to stabilize the market through supply and demand; however, price responsiveness for these critically necessary drugs is low in both short- and long-term markets. The reason for this low price responsiveness is the lack of alternatives coupled with the manufacturer’s ability to raise prices and not impact the consumer when the medication is linked to medical service (such as a physician office copay for service and medication administration). Additionally, limited shelf life and the costly equipment used to manufacture these products often impede the ability of generic companies to generate products in response to shortages in the short term.3
The FDA Has Taken Serious Actions
Rarely do we see politicians weighing in on drug industry issues. However, with the increasing frequency of drug shortages and the negative impact this issue has on patient care, President Barack Obama recently issued an executive order directing the FDA and the Department of Justice to take action to help reduce and prevent drug shortages, protect consumers, and prevent price gouging (TABLE 1).4 This action, which requires early notification of shortages, will help Congress achieve the goal of strengthening the ability of the FDA to prevent prescription drug shortages in the future. President Obama also sent a letter to manufacturers reminding them of their legal responsibility to report the discontinuation of certain drugs to the FDA. In addition, he encouraged companies to voluntarily notify the FDA about potential prescription drug shortages. Currently, such notification is not required. The president has released both a report developed by the Office of the Assistant Secretary for Planning and Evaluation (ASPE) of the Department of Health and Human Services that has evaluated the factors leading to drug shortages, and an FDA report on that agency’s role in monitoring, preventing, and responding to these shortages.4

Although an expanded manufacturing capacity is one clear solution to prevent shortages, this early notification can also have a meaningful impact on the occurrence and duration of shortages. Voluntary notification by a manufacturer would enable the FDA to immediately seek out other companies interested in producing the drug while working directly with the company to explore possible regulatory flexibility. Examples of this flexibility would include the extension of expiration dates and the hastening of the review for regulatory submissions.
Commissioner Hamburg stated that the FDA’s greatly expanded drug shortage team is notified of impending shortages and then contacts other manufacturers who have been “very responsive” when the FDA works with them to find ways to boost production.1 The FDA Drug Shortage Program (DSP) prioritizes products that have a significant effect on public health as a target of their efforts. This prioritization results from the DSP determination that a drug is “medically necessary,” which DSP defines as those drugs used to treat or prevent a serious disease or medical condition for which there are no alternative drugs available in adequate supply that are judged by medical staff to be an adequate substitute.3 Please refer to TABLE 2 for further clarification from the FDA.

Creative Solutions May Have Unintended Consequences
One proposal for a creative alternative to avoid drug shortages is for drug companies to explore international partnerships for the manufacture of drugs that are in short supply or are no longer available in the U.S. Production outside the country, however, can pose not only quality assurance issues, but may also create ethical dilemmas that can become the ultimate impasse. Recently, as part of a notice to the FDA to discontinue production of the anesthetic sodium thiopental, the U.S. manufacturer, Hospira Inc., communicated its decision to switch the manufacture of the drug from its North Carolina plant to a Hospira plant just outside Milan, Italy.5 However, the Italians were not willing to manufacture the product without a guarantee that it would not be used for lethal injections, which Hospira could not guarantee would be the case. Consequently, this medication shortage continues within the U.S.
In certain instances, the FDA has allowed emergency importation of a product from a foreign manufacturing source until the domestic shortage resolves. As of June 2011, the FDA had allowed six drugs to be imported under these conditions, four of which were oncology agents.2
Foreign importation is not a novel concept for drugs available for use in the U.S. In these cases, medications are not completely manufactured in the U.S. and may require key active ingredients to be produced within FDA-approved international sites. “Importation from approved sites outside of the U.S. may include importation of just the active ingredient and the final product is manufactured here, or there is importation of the total final product from sites outside of the U.S. that are either approved sources or unapproved sources. Unapproved sources have no FDA control, no oversight; therefore, importation of that generic or brand name product is illegal,” said Keith Webber, PhD, acting director of the Office of Generic Drugs.6
Previous efforts made by the FDA to require more documentation and compliance with rules, both for domestic and foreign manufacturers, have resulted in decisions to discontinue product lines rather than meet the FDA requirements. Prefilled epinephrine and propofol were recent casualties of manufacturer decisions to discontinue in lieu of addressing what they saw as overly bureaucratic requests of the FDA.7
Not Everybody Is Disadvantaged by This Drug Shortage Crisis
The FDA plans to work with the Department of Justice to investigate whether secondary wholesalers or other market entities have purchased drugs in short supply for purposes of “hoarding supplies” to then sell later at a higher price. A gray market, also known as a parallel market, is the trade of a commodity, in this case hoarded medication supplies, through distribution channels that, while legal, are unofficial, unauthorized, or unintended by the original manufacturer.7 According to President Obama’s Executive Order, “The ranking member of the House Committee on Oversight and Government Reforms, when announcing his investigation into these so-called gray markets, expressed concerns about a report that a leukemia drug whose typical contract price is about $12 per vial was being sold at $990 per vial—80 times higher. A Premier Healthcare Alliance Report released in August 2011 estimated that the typical gray market vendor marks up prices by an average of 650%. At the extreme, a drug used to treat high blood pressure that was normally priced at $25.90 was being sold at $1,200 due to a shortage.”4
Remedy May Come With Unintended Consequences
Some would argue that these corrective measures might come with unintended consequences. Notification of an impending shortage may exaggerate the problem of proactive purchasing and consumer stockpiling, as was seen with oseltamivir (Tamiflu), the antiviral that was recently the target of a demand that quickly exceeded the existing supply. In early 2005, Roche announced a production shortage due to demand in the midst of expanded use of oseltamivir during the H5N1 avian influenza epidemic in Southeast Asia in 2005. In response to the epidemic, various governments—including those of the United Kingdom, Canada, Israel, U.S., and Australia—stockpiled quantities of oseltamivir in preparation for a possible pandemic.8 Such stockpiling may only make the gray market worse. Another downside of such corrective measures is that some manufacturers and distributors do not have an allocation plan that would allow everyone to get “something” instead of allowing the stockpiling by those who order most aggressively and quickly in response to notifications.
The FDA Has No Distribution Authority
While shortages in brand-name medications have typically been less frequent, there is a new trend wherein brand-name companies contract with generic companies to manufacture older product lines to enable the production of newer agents. This “subcontracting” may be responsible for the brand-name shortages of recent years, including that of the injectable chemotherapeutic agent doxorubicin (Doxil).9 This contract manufacturing is considered proprietary information and not public record, so it is difficult, if not impossible, to predict whether certain brand names will be at risk of shortage in the future. Companies may also decide to simply discontinue production of a trade-name drug once it goes off patent, leaving a strain in supply until a generic company manufactures the product.2
Some experts have proposed additional regulations to assist during shortages, such as2,9:
- Requiring notification of a pending shortage, which is now voluntary
- Enforcing penalties for those who do not follow the regulations
- Developing practice guidelines for shortages—what drug would be “next best”; often a delay may be better than a suboptimal drug
- Moving forward with the extension of expiration dates through regulatory flexibility
- Incentivizing production for companies willing to manufacture, despite low profit margin
- Rewarding companies who do not have shortages
- Creating a federal stockpile of “medically necessary drugs.”
Impact on Patients and Prescribers
Shortages create the potential for significant, even life-threatening risk for patients by delaying or withholding essential medical treatments. Not only do these shortages represent a barrier to providing appropriate care, but they also create a significant risk for an increase in medication errors. The required dose conversion from IV to oral therapy can lead to serious, if not fatal, medication errors.10 The risk becomes even greater when factoring in medication errors attributed to unfamiliar gray market products.
Some experts speculate that drug shortages cost U.S. hospitals at least $200 million annually, and that health care providers pay an average of 11% more for drugs in short supply. When considering the impact of professional staff time to manage the shortages and to explore alternative treatment options, this cost increases exponentially.10
“These drug shortages can create a significant burden on practitioners. The shortage crisis is not resolving. Pharmacists are spending more time trying to track down medications than ever, leaving less time to communicate the importance of adhering to medication therapy,” stated Lawrence H. Mokhiber, MS, RPh, executive secretary of the New York State Board of Pharmacy and past president and chair of the National Association of Boards of Pharmacy (interview, February 15, 2012). “Further complicating the issue is that patients may be more likely to seek their own remedy to shortages and turn to the Internet and imported medications, creating a huge risk of patient harm if the patient obtains a supply of adulterated or imposter drug. The public may also misdirect their frustration about these shortages as a problem created by the pharmacy, instead of the public issue that it truly is…I am concerned both for the public and pharmacy professionals who care for them.”
Physicians are also adversely affected by reduced treatment options for patients. For example, Jeffrey Schratz, MD, general surgeon and former chief of surgery, Eastern Niagara Hospital System, describes his frustration when effective medicines exist but are unavailable for reason of drug shortage. “A classic example is a 2 AM phone call from a nurse regarding a post-op surgical patient who is nauseated. Zofran is an excellent medicine to relieve their nausea, but I am often told there is none available when it is needed. This is really frustrating and seems unfair to patient and provider alike,” stated Dr. Schratz (interview, February 15, 2012).
For patients, these shortages can induce a great deal of frustration, even for those shortages that do not represent a life-and-death treatment turning point. However, in cases such as the recent methotrexate shortage, a crucial medication for children with a type of leukemia and for high-dose treatment of bone cancer, the stress and fear of the negative outcomes of shortage are indescribable.1
Critical Medications
Many practitioners and patients may argue that a critical medication shortage can only be defined as a deficit of “life-saving” medications. But in the case of psychiatric illness, ongoing medication treatment can be a necessary component of maintaining stability. “Discontinuities in treatment are a major cause of psychiatric crises and hospital readmissions that can be traumatic and costly. Adherence to ongoing treatment is difficult enough; shortages of essential psychiatric medications, such as the injectable, long-acting antipsychotic fluphenazine decanoate, are unacceptable,” stated Michael Hogan, PhD, commissioner of the New York State Office of Mental Health (interview, February 12, 2012).
“Some of these medication shortages can bridge more than one specialty practice setting, such as the case of the injectable anxiolytic lorazepam. This product is used to provide rapid anxiety relief for patients with psychiatric exacerbation, but is also used routinely in severe alcohol withdrawal, IV sedation during procedures, and other critical care interventions, with few equally safe and effective alternatives,” said Dr. Schratz (interview, February 15, 2012).
While there have been regulations to protect people on the most critical “medically necessary” drugs, the regulation requiring a 6-month notice before permanent discontinuation lacks true value, as the manufacturer is the source of the definition of “medical necessity” for the products it produces.2
Conclusion
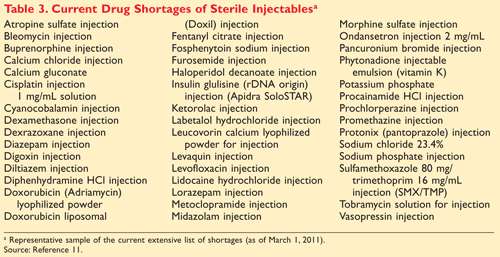
Pharmacists will continue to be the front-line defense and patient partner in addressing the complications of drug shortages. It is key that pharmacists have access to real-time, accurate drug shortage information. A selected list of current drug shortages of sterile injectables is provided in TABLE 3.11 Addressing shortages requires collaboration and guidelines to manage patient care, especially in the midst of highly specialized medication regimens. Even the FDA acknowledges that drug shortages will continue to be a clinical concern in the future. “I don’t think we can ever close the book on drug shortages,” Commissioner Hamburg stated. “I think we will always have to be vigilant.”1
REFERENCES
1. Associated Press. FDA: New suppliers to ease 2 cancer drug shortages. CNS News. February 22, 2012. http://cnsnews.com/news/article/fda-new-suppliers-ease-2-cancer-drug-shortages. Accessed May 11, 2012.
2. Ventola CL. The drug shortage crisis in the United States: causes, impact, and management strategies. P T. 2011;35:740-757.
3. Economic analysis of the causes of drug shortages. ASPE Issue Brief. October 2011. http://aspe.hhs.gov/sp/reports/2011/DrugShortages/ib.shtml. Accessed February 28, 2012.
4. Fact sheet: Obama administration takes action to reduce prescription drug shortages in the U.S. The White House.
October 31, 2011.
www.whitehouse.gov/the-press-office/2011/10/31/fact-sheet-obama-administration-takes-action-reduce-prescription-drug-sh.
Accessed February 28, 2012.
5. Hospira statement regarding Pentothal (sodium
thiopental) market exit. News release. January 21, 2011.
http://phx.corporate-ir.net/phoenix.zhtml?c=175550&p=irol-newsArticle_print&ID=1518610&highlight.
Accessed May 11, 2012.
6. Demler TL. The generic drug industry: current issues. US Pharm. 2011;36(6)(Generic suppl):36-40. www.uspharmacist.com/content/s/167/c/28737/. Accessed February 28, 2012.
7. Stein R. Shortages of key drugs endanger patients. Washington Post.
May 1, 2011.
www.washingtonpost.com/national/shortages-of-key-drugs-endanger-patients/2011/04/26/AF1aJJVF_story.html.
Accessed February 28, 2012.
8. Tamiflu—oseltamivir production. www.news-medical.net/health/Tamiflu-Oseltamivir-Production.aspx. Accessed May 3, 2012.
9. Sahw G. Oncology drug shortage worsens; 'mayhem' cited. Pharmacy Practice News. November 2011. www.pharmacypracticenews.com. Accessed May 11, 2012.
10. Eastman P. Oncology drug shortages worsening, threatening patient care. Oncol Times. 2011;33:14-15.
11. Current drug shortages. FDA. www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050792.htm. Accessed March 1, 2012.
To comment on this article, contact rdavidson@uspharmacist.com.






