US Pharm. 2012;37(7)(Oncology suppl):3-7.
ABSTRACT: Breakthrough pain (BTP), a common problem in patients with cancer or other chronic pain syndromes, is a transitory increase in pain that occurs despite relatively stable controlled background pain. BTP can impact the quality of life, sleep, mental health, and overall well-being of the patient. Therapy should be individualized with continuous reassessment of pain severity and etiology. Newer opioid analgesic formulations such as transmucosal immediate-release fentanyl (TIRF) provide multiple options for the management of BTP.
Breakthrough pain (BTP) is a common problem in patients with cancer pain or other chronic pain syndromes. While there is no official definition, it can be summarized as a transitory or brief increase in pain that occurs despite relatively stable controlled background pain, as in a patient managed with a long-acting or extended-release opioid analgesic. It is important to note that for pain to be categorized as BTP, management of background pain should be in place. This ensures that BTP is not confused with, or treated as if it were, uncontrolled pain.1-3 The prevalence of BTP is highly variable based on the setting; outpatients and inpatients admitted with uncontrolled pain have a reported prevalence of 70% to 95%.3-5 Moreover, BTP can impact the quality of life, sleep, mental health, and overall well-being of the patient. With proper identification, BTP can be appropriately managed with rational treatment strategies that provide rapid analgesia.
Classification of BTP
BTP can be classified by several definitions. Incident pain is caused by an evident precipitating cause or event. This type of BTP is usually predictable and is most commonly caused by movement. Spontaneous or idiopathic pain does not have an identifiable precipitating event. End-of-dose failure, which is described as an exacerbation of pain that occurs prior to the next dose of a background analgesic, is no longer considered a subtype of BTP and is believed to be representative of inadequate analgesia. However, this is a common issue that can occur due to a reduction in serum analgesic levels as a result of inadequate dosing or an inappropriate dosing interval. When this occurs, adequate pain management cannot be sustained and the patient can experience a flare of pain.6
Pain Assessment and Screening
Proper pain assessment and screening are essential to the management of BTP. First, it is important to establish that the pain is actually due to BTP. TABLE 1 lists three questions that are useful in identifying BTP.1,6 If it is determined that the patient does have BTP, it is recommended that a comprehensive assessment be conducted prior to treatment initiation.7 This assessment should include, but is not limited to, several of the following:
1) Evaluation and identification of the pain syndrome.
2) Evaluation of the functional status of the patient.
3) Psychological evaluation to detect any possible disturbances.
4) A current and past medical history to identify any additional disease states.
5) Substance abuse history.

Overall, appropriate assessment and screening will allow for proper identification and classification of the pain syndrome. This allows practitioners to differentiate between patients with subtherapeutic background pain due to inadequate dosing of an analgesic and patients with pain due to a transient exacerbation that occurs even though the patient is normally adequately controlled on a background pain regimen.
Goals of Therapy
The goals of therapy for the management of BTP include providing rapid analgesia with medications that are appropriate based on pain severity, improving/maintaining function, and minimizing adverse effects from therapy. Special attention should be paid to the fact that certain pain syndromes, particularly in the setting of cancer pain, can be highly unpredictable and may require continual reassessment of pain.
Pharmacologic Management of BTP
The typical management paradigm for pain is based on providing an adequate amount of background analgesia while providing the patient with the appropriate amount of medication for breakthrough events. Current practice guidelines, including the National Comprehensive Cancer Network (NCCN) Adult Cancer Pain Guidelines, do not offer specific recommendations regarding agents or doses for the treatment of BTP, but do recommend titrating to a dose of medication that relieves the patient’s breakthrough pain throughout the dosing period without causing unmanageable adverse effects.8-10 If possible, the same agent should be used for BTP as for background therapy. When increasing the background analgesic dose in the setting of opioid use, calculations should be based upon the total daily opioid dose over the past 24 hours including the patient’s as-needed medication. It is generally recommended that the dose not be increased more than 30% to 50% at a time.7 While optimizing the dose of opioids is extremely important, consideration should also be given to the route of administration (TABLE 2).
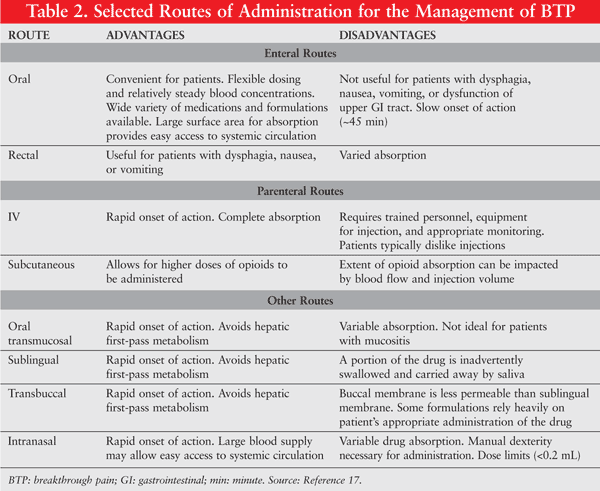
Opioid Analgesics: Opioids are generally considered to be the agents of choice when treating moderate-to-severe chronic pain, and these agents exert their effects through the activation of opioid receptors within the brain and spinal cord, resulting in pain modulation and analgesia.7 While opioids are considered to be equianalgesic, some have different pharmacokinetic profiles that make them ideal agents to be formulated as analgesics with a rapid onset of action. For example, fentanyl is highly lipophilic and is now available in numerous transmucosal formulations that have the following advantages for use in BTP: 1) rapid onset of analgesia (usually within minutes); 2) ease of use for patients; 3) easy adjustment of dose; and 4) ability to combine well with other medications used for management of background pain.11-15
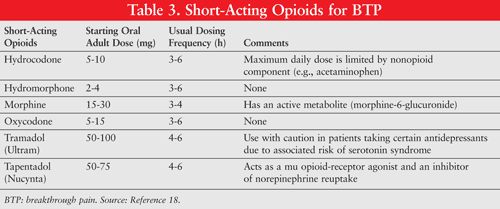
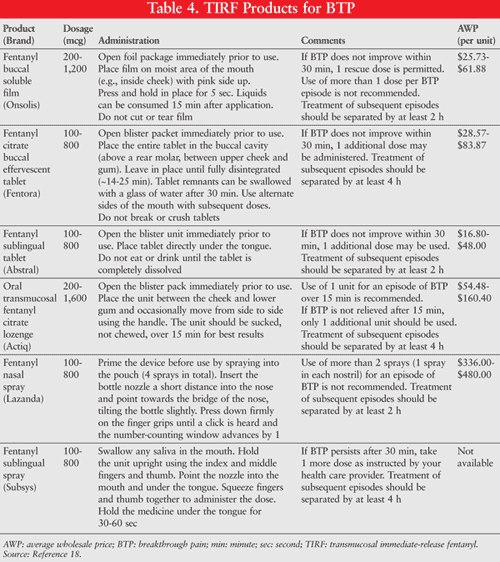
BTP events may be unpredictable but typically reach peak severity within 5 minutes, and have an average duration of 30 minutes, thereby emphasizing the importance of the use of analgesics with a rapid onset and short duration of action.2,4 Trials comparing immediate-release oral morphine to transmucosal immediate-release fentanyl (TIRF) have shown that patients taking TIRF have improved pain intensity scores and pain relief.16 While these new medications have proven to be very useful in the treatment of BTP, it is important that patients receive adequate counseling on the appropriate use of these medications, as each TIRF product has unique features. For example, patients using the Oral Transmucosal Fentanyl Citrate (OTFC) lozenge must be educated that they cannot chew or swallow the lozenge. If patients mistakenly chew or swallow this dosage form, a substantial portion of the medication could be subjected to first-pass metabolism and destroyed.17 When prescribing or dispensing a new TIRF product, pharmacists should determine that the patient is able to demonstrate that he or she can properly use the prescribed dosage form to ensure adequate delivery of the medication. TABLES 3 and 4 list the short-acting opioid analgesics and TIRF products that are commonly used for the management of BTP.18
REMS for TIRF: To ensure the safe and effective use of TIRF, the FDA has approved a single, shared Risk Evaluation and Medication Strategy (REMS) called the TIRF REMS Access Program (www.TIRFREMSaccess.com), which began in March 2012.19 The goals of this REMS program are to ensure patient access to these medications and to mitigate the risk of misuse, abuse, addiction, overdose, and complications due to medication errors. Prescribers of TIRF products for outpatient use are required to enroll in the TIRF REMS Access Program by reviewing the education program, successfully completing the knowledge assessment, and completing an enrollment form, with reenrollment required every 2 years.19
Both outpatient and inpatient pharmacies that dispense TIRF products are required to enroll in the program through the designation of an authorized pharmacist who must undergo the same enrollment process as prescribers before complete enrollment can be made on behalf of the pharmacy. The authorized pharmacist will then train other pharmacy staff in the appropriate dispensing of TIRF products. Patients are required to sign a patient-provider agreement and will be provided with a medication guide. Prescribers and patients are not expected to enroll in this program when TIRF products are used in inpatient settings, unless the patient is in a facility (e.g., long-term care, hospice) where medications are obtained from an outpatient pharmacy. Some of the TIRF products already have a REMS program in place, and prescribers and pharmacies that are enrolled in one of these programs will automatically be transitioned to the new TIRF REMS Access Program.19
The TIRF REMS Access Program addresses a variety of issues that are focused on ensuring the safe use of TIRF products in the appropriate patient population. The four main goals of the TIRF REMS Access Program are19:
1) Prescribing and dispensing TIRF medicines only to appropriate patients, which includes use only in opioid-tolerant patients.
2) Preventing inappropriate conversion between fentanyl products.
3) Preventing accidental exposure to children and others for whom the product was not prescribed.
4) Educating prescribers, pharmacists, and patients on the potential for misuse, abuse, addiction, and overdose.
In the TIRF REMS Access Program, the medications are indicated for the management of BTP in adult patients with cancer who are aged 18 years or older and who are already receiving and are tolerant of regular opioid therapy for underlying, persistent cancer pain. The only exception is for Actiq and its generic fentanyl equivalents, which are approved for cancer patients aged 16 years and older. TIRF medications are not interchangeable with each other, and pharmacists are responsible for verifying dosages and proper dose conversion. TIRF products contain fentanyl, a Schedule II controlled substance. Therefore, appropriate measures should be taken to minimize the risk of abuse, misuse, addiction, and overdose. Pharmacists should be involved in appropriate patient counseling with patients on TIRF medications and should review the product-specific medication guide with patients and caregivers.19
Overall, TIRF medications offer a new, innovative delivery system to assist with the management of BTP in cancer patients. All patients receiving TIRF products require careful monitoring, and pharmacists play a very important role in dispensing and monitoring patients on these medications.
Nonpharmacologic Management Options
While pharmacologic management is standard in the treatment of BTP, there are other, nonpharmacologic methods that may be useful. Massage therapy, application of heat or cold, distraction, and relaxation techniques have been suggested as possible adjunctive treatments for BTP.6 Patients may prefer to try these techniques before or during pharmacologic management; however, there is little evidence to support their efficacy in the treatment of BTP.
Summary
BTP is a common issue among patients with cancer pain and other chronic pain syndromes. Due to interpatient variability, therapy for BTP should be individualized with continuous reassessment of pain severity and etiology. The emergence of newer opioid analgesic formulations provides multiple options for BTP management; however, the clinical utility of these products may be limited due to cost. Pharmacists can play an important role in the management of BTP by assisting with patient assessment, product selection, and continuous monitoring of efficacy and adverse reactions related to the chosen therapies.
REFERENCES
1. Mercadante S. Managing breakthrough pain. Curr Pain Headache Rep. 2011;15:244-249.
2. Davies AN. The management of breakthrough cancer pain. Br J Nurs. 2011;20:803-807.
3. Mercadante S, Zagonel V, Breda E, et al. Breakthrough pain in oncology: a longitudinal study. J Pain Symptom Manage. 2010;40:183-190.
4. Burton B, Zeppetella G. Assessing the impact of breakthrough cancer pain. Brit J Nurs. 2011;20:S14-S19.
5. Hauegen DF, Hjermstad MJ, Hagen N, et al. Assessment
and classification of cancer breakthrough pain: a systematic literature
review. Pain. 2010;149:476-482.
6. Zeppetella G. Breakthrough pain in cancer patients. Clin Oncol. 2011;23:393-398.
7. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695-1700.
8. National Comprehensive Cancer Network. NCCN clinical
practice guidelines in oncology. Adult cancer pain. V.1.2009.
www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed February
15, 2012.
9. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Davies AN, Dickman A, Reid C, et al. The management of
cancer-related breakthrough pain: recommendations of a task group of
the Science Committee of the Association for Palliative Medicine of
Great Britain and Ireland. Eur J Pain. 2009;13:331-338.
11. Mystakidou K, Panagiotou I, Gouliamos A. Fentanyl nasal spray for the treatment of cancer pain. Expert Opin Pharmacother. 2011;12:1653-1659.
12. Fine PG, Messina J, Xie F, Rathmell J. Long-term
safety and tolerability of fentanyl buccal tablet for the treatment of
breakthrough pain in opioid-tolerant patients with chronic pain: an
18-month study. J Pain Symptom Manage. 2010;40:745-760.
13. Chwieduk CM, McKeage K. Fentanyl sublingual in breakthrough pain in opioid-tolerant adults with cancer. Drugs. 2010;70:2281-2288.
14. Zeppetella G, Messina J, Xie F, Slatkin NE. Consistent
and clinically relevant effects with fentanyl buccal tablet in the
treatment of patients receiving maintenance opioid therapy and
experiencing cancer-related breakthrough pain. Pain Practice. 2010;10:287-293.
15. Mercadante S, Ferrera P, Arcuri E. The use of fentanyl
buccal tablets and breakthrough medication in patients receiving
chronic methadone therapy: an open label preliminary study. Support Care Cancer. 2010;19:435-438.
16. Coluzzi PH, Schwartzberg L, Conroy J, et al.
Breakthrough cancer pain: a randomized trial comparing oral transmucosal
fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR). Pain. 2001;91:123-130.
17. Nicholson B, Agarwala SS. Opioid delivery in the
treatment of cancer breakthrough pain: a review of routes of
administration. J Opioid Manage. 2011;7:69-79.
18. Micromedex Healthcare Series [Internet database].
Greenwood Village, CO: Thomson Reuters; 2012. www.micromedex.com.
Accessed February 15, 2012.
19. TIRF REMS Access Program. Updated 2012. www.TIRFREMSaccess.com. Accessed April 15, 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





