US Pharm. 2012;37(9)(Oncology suppl):15-18.
Over the last four decades, rates of cervical cancer have shown a steady decline of almost 70%. This is largely attributed to the introduction of the Papanicolaou (Pap) test, which can detect cervical changes in their earliest, most curable stage.1 Although rates continue to drop by nearly 3% every year, an estimated 12,200 new cases and 4,200 deaths occurred in 2010. A disparity among races has been noted, with Hispanic and African American women having the highest rates of cervical cancer. This can be explained mostly by low socioeconomic status, with its attendant problems of less education, less health coverage, and greater prevalence of behavioral risk factors.
Most troubling are the rates in developing countries. Worldwide, cervical cancer is still the second most common malignancy in women. The same contributing factors—lack of education and access to screenings—are to blame. Furthermore, there has been little progress in the treatment of cervical cancer. Clearly, effective strategies must be developed that target both the prevention and the treatment of cervical cancer.2
PRESENTATION
Cervical cancer develops slowly, providing more opportunities for early detection and prevention. Its progression from precancerous changes in the lining of the cervix (known as dysplasia) to invasive stages of cancer can take years. Squamous cell carcinoma (SCC) and adenocarcinoma are the predominant types of cervical cancer, with SCC comprising 85% of cases. Cervical cancer frequently appears in midlife, with the majority of cases diagnosed after age 30 years; it is rarely detected in women younger than 20 years.1,2
Initially, patients are usually asymptomatic, with symptoms appearing only as the disease advances. Common symptoms include abnormal vaginal bleeding (frequently postcoital), pain during intercourse, and unusual vaginal discharge.1,2
SCREENING AND DIAGNOSIS
The value of routine screenings in the prevention of cervical cancer cannot be overstated. The incidence and mortality of cervical cancer have decreased at least 80% since the advent of regular Pap testing. Accordingly, the American College of Obstetricians and Gynecologists recommends yearly Pap testing 3 years after a woman begins having vaginal intercourse, but no later than age 21 years. At age 30 years, this interval can be reduced to every 2 to 3 years in women with normal Pap tests. Women who are vaccinated against human papillomavirus (HPV) are not protected against all types of HPV that cause cervical cancer; therefore, they should continue to receive routine Pap testing. Additional screening has no benefit in women older than 60 years with a history of negative tests.3
Additional testing or biopsy is indicated for a positive Pap test denoting squamous intraepithelial lesions or atypical glandular cells. An HPV DNA test is used to determine whether a patient is currently infected with HPV (it does not provide information on past infections).1,3 The test has been integrated into current screening guidelines to triage women with atypical findings.
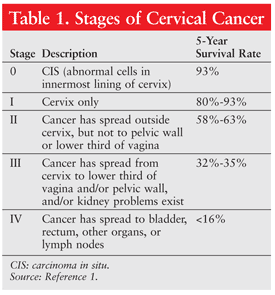
A positive biopsy warrants further testing with the conventional methods for diagnosing cancer—mainly imaging studies (CT, MRI, positron emission tomography). Once cancer has been confirmed, staging is performed to guide treatment protocols. TABLE 1 summarizes the different stages of cervical cancer and the prognosis based on 5-year survival rates.
RISK FACTORS
HPV is undoubtedly the single most important contributor to the development of cervical cancer. Other risk factors are indirectly associated with HPV by increasing a woman’s exposure to the virus. TABLE 2 lists the most common risk factors for cervical cancer.1,3

HPV
The FDA approved Gardasil, a new quadrivalent vaccine for preventing infection from four types of HPV (types 6, 11, 16, and 18), in 2006.4 Cervarix, a bivalent vaccine for preventing HPV types 16 and 18, was approved in 2009. HPV types 16 and 18 are responsible for approximately 70% of cervical cancer cases worldwide; HPV 6 and HPV 11 account for nearly 90% of cases of genital warts.5
More than 40 types of HPV are transmitted sexually. For this reason, maximum benefit is achieved only if the vaccine is administered before a person becomes sexually active or has been exposed to HPV.6 This corresponds to the age groups that have been approved to receive Gardasil (9-26 years) and Cervarix (10-25 years). Currently, only Gardasil is approved for both females and males. The effects of these vaccines in individuals already infected with HPV are still being investigated.7 The Advisory Committee on Immunization Practices recommends that a woman with an abnormal Pap test who is in the appropriate age group receive an HPV vaccination to protect against high-risk HPV types she could still be exposed to.8 The quadrivalent vaccine has been proven safe and effective for women older than 25 years, and several countries have approved it for this age group.9 Both vaccines are formulated for administration in three doses over a 6-month period, although two doses may be equally effective (currently under investigation).
The most common adverse effect (AE) of the vaccines is local injection-site reactions. Gardasil had a slightly higher incidence of syncope and venous thrombotic events compared with other vaccines. As a precaution, the FDA recommends that vaccine recipients remain seated or lying down and be monitored for 15 minutes after vaccination to prevent syncope-related falls.10
TREATMENT
Approved treatment options for cervical cancer include surgery, radiotherapy (RT), and chemotherapy. Targeted therapies are currently under investigation. Several factors influence the choice of treatment: tumor size, cancer stage, and the patient’s age. The patient’s wish to maintain fertility or sexual activity also must be taken into consideration.1,3
Surgery and RT
Surgery and RT are effective for carcinoma in situ and cancers that are confined to the cervix (stage I). Surgery is the preferred treatment at this point. There are several procedures for removing SCC in situ, including laser surgery, cryosurgery, and the loop electrosurgical excision procedure (involving high-frequency electrical currents). Conization, also known as cone biopsy, is an important tool for the accurate diagnosis of cervical lesions and the treatment of localized cancer cells. In this procedure, a cone-shaped piece of tissue is removed to confirm the presence of cancerous cells.1,3
Adenocarcinomas and stage I cancers that have spread to the uterus require hysterectomy, in which the uterus and cervix are removed. In radical hysterectomy, the upper part of the vagina is removed also. Trachelectomy leaves the uterus intact, offering an alternative for women who want to avoid the infertility resulting from hysterectomy.1,11
RT is a critical component in the treatment of cervical cancers. It is associated with cure rates of 75% to 80%, comparable to radical hysterectomy. Both treatments show identical 5-year overall survival (OS) and disease-free survival rates.3 As monotherapy, RT is reserved for stage I and early stage II malignancies in patients with intact cervical cancer that is not amenable to surgery. Following hysterectomy, RT is indicated when other risk factors (e.g., large tumor size, positive surgical margins) are present that could increase the chance of residual cancer cells. Both surgery and RT are associated with infertility; however, radiation also causes premature menopause, which negatively affects the patient’s sex drive. Therefore, surgery is the preferred treatment for patients wishing to preserve sexual activity.2
Chemotherapy
Concurrent chemotherapy and RT have been shown to significantly improve patient survival, beginning with stage IB disease. Chemoradiotherapy (CRT) also is considered in early stage I cancers in patients who are not candidates for surgery. With the exception of platinum-based agents, cytotoxic chemotherapy yields marginal improvement in response rates of patients with cervical cancer. For this reason, the search continues for new combinations of chemotherapeutic agents for cervical cancer.2
Cisplatin: Cisplatin-based CRT was the first treatment to have a considerable impact on survival outcomes in cervical cancer patients. This approach was established as the primary treatment for locally advanced cervical cancer in stages IB2, II, III, and IV. The National Comprehensive Cancer Network (NCCN) incorporated this combination into its guidelines after analyzing the results of five randomized phase III clinical trials. In the trials, RT was compared with cisplatin alone, cisplatin plus 5-fluorouracil (5-FU), or hydroxyurea. Each trial included several hundred patients, and all participants had invasive cervical cancer.12 The trials showed a 30% to 50% reduction in mortality risk for CRT compared with RT alone. Trial outcomes also favored the addition of 5-FU to cisplatin-based CRT, although increased toxicity was observed.2 Long-term follow-up of three trials showed improved progression-free survival (PFS) and OS in the cisplatin-based CRT group versus the RT monotherapy and hydroxyurea combination groups.13 The most commonly used cisplatin regimen is 40 mg/m2.14
Topotecan: A breakthrough occurred when a phase III trial found topotecan to be the first new combination therapy to significantly prolong survival. The trial involved 293 patients with stage IVB recurrent or persistent cervical cancer who were randomized to topotecan plus cisplatin or cisplatin alone. Median OS was 6.4 months for cisplatin monotherapy versus 9.4 months for cisplatin plus topotecan.15 The combination group had a moderate increase in AEs, specifically bone marrow suppression, but quality of life was maintained. In 2006, the FDA approved a topotecan-cisplatin combination for recurrent or persistent cervical cancer.16 In an effort to ameliorate dose-limiting hematologic toxicities, a phase I trial of different topotecan dosing regimens is being conducted.17
Paclitaxel: After a promising phase II trial, the Gynecologic Oncology Group conducted a phase III review of cisplatin combined with paclitaxel in 264 patients with stage IVB recurrent or persistent cervical cancers. The combination group had increased response rates compared with the cisplatin-only group (36% vs. 19%, respectively); it also showed an increase in PFS (4.8 mo vs. 2.8 mo), but not median survival. The cisplatin-paclitaxel group experienced more neutropenia, anemia, and neuropathy, but overall quality of life was maintained. Based on favorable outcomes when carboplatin was substituted for cisplatin, results of a small phase II trial that combined carboplatin and paclitaxel encouraged further investigation in a phase III trial.18 In practice, many physicians use the carboplatin-paclitaxel combination because of ease of administration, tolerability, and preservation of renal function. Platinum-based paclitaxel combinations also have less toxicity and are easier to administer than cisplatin and topotecan.2,19
Gemcitabine: Gemcitabine, which was studied in combination with cisplatin, is another option for first-line combination therapy for locally advanced cervical cancer. A phase III, open-label, randomized trial compared PFS in 515 patients with stage IIB-IVA cervical cancer. Patients received either concurrent cisplatin and RT or a combination of cisplatin and gemcitabine with adjuvant RT. PFS improved significantly in the gemcitabine group (74.4% vs. 65%), including OS (78% vs. 69%) and time to progression. A notable increase in toxicity occurred in the combination group compared with the standard-treatment group (86.5% vs. 46.3%, respectively), although AEs were clinically manageable.20,21
Toxicities: The use of chemotherapy is often limited by toxic AEs. Bone marrow suppression, neuropathies, and severe nausea and vomiting can be minimized with appropriate monitoring and effective use of antiemetics. Additionally, patients receiving cisplatin should be adequately hydrated to prevent renal toxicity. Drug interactions that can potentiate cisplatin’s ototoxic and nephrotoxic effects should be avoided. Platinum agents and paclitaxel are commonly associated with hypersensitivity reactions. Patients should be observed for signs of allergic reaction (e.g., erythroderma, tachycardia, chest tightness, wheezing, facial swelling, hypotension). Appropriate dose reductions and chemotherapy modifications should be undertaken depending upon the toxicities experienced and the therapeutic goals.22
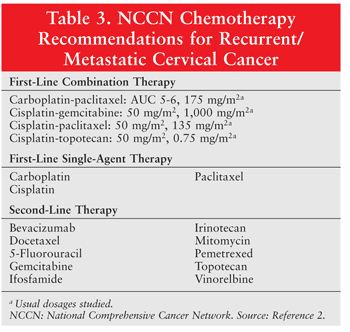
TABLE 3 summarizes the NCCN’s chemotherapy guidelines for advanced cervical cancer.2
Targeted Therapies
Advanced (stage IVB) and recurrent cervical carcinomas have a poor prognosis, with a median survival of 9 to 10 months and a 5-year survival rate of 15% to 35%.1,23 Treatment at this stage is palliative. Current phase III trials are attempting to establish which platinum-doublet regimen is optimal in this setting. The success of targeted therapies in the treatment of specific cancers has led to their increasing investigation as an addition to standard chemotherapy regimens.
An integral part of cancer metastasis is angiogenesis, a process through which the tumor creates additional blood vessels to sustain growth.24 HPV infections are linked to high levels of vascular endothelial growth factor (VEGF), a signaling protein responsible for initiating angiogenesis. Since cervical cancers depend upon angiogenesis for tumor survival, the use of antiangiogenesis-targeted therapies is seen as a next approach for recurrent and advanced cervical cancer.
Another factor that has sparked the interest of researchers is the presence of epidermal growth factor receptors (EGFRs) on the surface of cervical cancer tumor cells. EGFRs are tyrosine kinases (TKs), and the signaling of these proteins initiates a molecular cascade that leads to cell proliferation, invasion, and migration.24,25 Inactivation of these TKs is a promising approach that has led to several clinical trials. The biologics cetuximab, pazopanib, lapatinib, and bevacizumab have been investigated for the treatment of cervical cancer. To date, only bevacizumab has shown positive outcomes warranting further trials.23
Potential Therapies
The National Cancer Institute (NCI) is concentrating its research efforts on the long-term safety of existing vaccines, duration of protection, and prevalence of infection with other HPV types. Another important focus for the NCI is the development of a new generation of vaccines that would be both preventive and therapeutic, treating women who previously were infected with HPV. Clinical trials of carrageenan, a topical microbicide extracted from seaweeds that has inhibited HPV infection in laboratory studies, are being conducted. Most recently, it has been suggested that the protease inhibitor lopinavir could become a new form of treatment for cervical cancer. Lopinavir selectively targets HPV-infected cells to produce an antiviral protein, ribonuclease L, which allows the body to recognize and destroy the virus. Effective doses would be 10 to 15 times the concentration used in HIV patients, so topical application would be necessary.26 Ultimately, prevention is the most effective approach; therefore, scientists are also working on faster and more accurate methods for detecting HPV.10
REFERENCES
1. American Cancer Society. Cervical cancer: prevention and early
detection.
www.cancer.org/Cancer/CervicalCancer/MoreInformation/CervicalCancerPreventionandEarlyDetection/cervical-cancer-prevention-and-early-detection-what-is-cervical-cancer.
Accessed October 11, 2011.
2. National Comprehensive Cancer Network. NCCN clinical practice
guidelines in oncology. Cervical cancer. Version 1.2012.
www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed
October 13, 2011.
3. National Cancer Institute. Pap and HPV testing.
www.cancer.gov/cancertopics/factsheet/detection/Pap-test. Accessed July
24, 2012.
4. FDA. Gardasil. www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042. Accessed July 24, 2012.
5. FDA. FDA approves new vaccine for prevention of cervical cancer.
www.fda.gov/newsevents/newsroom/pressannouncements/ucm187048.htm.
Accessed July 24, 2012.
6. Hildesheim A, Herrero R, Wacholder S, et al. Effect of human
papillomavirus 16/18 L1 viruslike particle vaccine among young women
with preexisting infection: a randomized trial. JAMA. 2007;298:743-753.
7. National Cancer Institute. HPV vaccine not effective for treating
pre-existing infections.
www.cancer.gov/clinicaltrials/results/summary/2007/hpv0807. Accessed
July 24, 2012.
8. CDC. FDA licensure of bivalent human papillomavirus vaccine (HPV2,
Cervarix) for use in females and updated HPV vaccination
recommendations from the Advisory Committee on Immunization Practices
(ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
9. Castellsagué X, Schneider A, Kaufmann AM, Bosch FX. HPV
vaccination against cervical cancer in women above 25 years of age: key
considerations and current perspectives. Gynecol Oncol. 2009;115(suppl 3):S15-S23.
10. National Cancer Institute. Human papillomavirus (HPV) vaccines.
www.cancer.gov/cancertopics/factsheet/prevention/HPV-vaccine. Accessed
July 24, 2012.
11. Beiner M, Covens A. Surgery insight: radical vaginal
trachelectomy as a method of fertility preservation for cervical cancer.
Nat Clin Pract Oncol. 2007;4:353-361.
12. National Institutes of Health. (1999, Feb 22). NCI issues
clinical announcement on cervical cancer: chemotherapy plus radiation
improves survival. www.nih.gov/news/pr/feb99/nci-22.htm. Accessed July
24, 2012.
13. Rose PG, Ali S, Watkins E, et al. Long-term follow-up of a
randomized trial comparing concurrent single agent cisplatin,
cisplatin-based combination chemotherapy, or hydroxyurea during pelvic
irradiation for locally advanced cervical cancer: a Gynecologic Oncology
Group Study. J Clin Oncol. 2007;25:2804-2810.
14. Small W Jr. Combining Targeted Biological Agents with Radiotherapy: Current Status and Future Directions. New York, NY: Demos Medical Publishing; 2008.
15. Long HJ III, Bundy BN, Grendys EC Jr, et al. Randomized phase III
trial of cisplatin with or without topotecan in carcinoma of the
uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005;23:4626-4633.
16. Robati M, Holtz D, Dunton CJ. A review of topotecan in combination chemotherapy for advanced cervical cancer. Ther Clin Risk Manag. 2008;4:213-218.
17. FDA. FDA approves first drug treatment for late-stage cervical
cancer.
www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108672.htm.
Accessed July 24, 2012.
18. Markman M. Update on endometrial and cervical cancer. Medscape
Education. www.medscape.org/viewarticle/481475. Accessed July 24, 2012.
19. Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of
cisplatin with or without paclitaxel in stage ivb, recurrent, or
persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology
Group Study. J Clin Oncol. 2004;22:3113-3119.
20. Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III,
open-label, randomized study comparing concurrent gemcitabine plus
cisplatin and radiation followed by adjuvant gemcitabine and cisplatin
versus concurrent cisplatin and radiation in patients with stage IIB to
IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678-1685.
21. National Cancer Institute. Treatment regimen extends survival for
women with cervical cancer.
www.cancer.gov/clinicaltrials/results/summary/2009/gemcitabine_cervical0609.
Accessed July 24, 2012.
22. Gahart BL, Nazareno AR. 2007 Intravenous Medications: A Handbook for Nurses and Health Professionals. 23th ed. St. Louis, MO: Mosby Elsevier; 2007.
23. Monk BJ, Sill MW, Burger RA, et al. Phase II trial of bevacizumab
in the treatment of persistent or recurrent squamous cell carcinoma of
the cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2009;27:1069-1074.
24. Gerber D. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311-319.
25. National Cancer Institute. Metastasis requires angiogenesis.
www.cancer.gov/cancertopics/understandingcancer/angiogenesis/page2.
Accessed July 24, 2012.
26. Batman G, Oliver AW, Zehbe I, et al. Lopinavir up-regulates
expression of the antiviral protein ribonuclease L in human
papillomavirus-positive cervical carcinoma cells. Antivir Ther. 2011;16:515-525.
To comment on this article, contact rdavidson@uspharmacist.com.





