US Pharm. 2012;37(11)(Oncology suppl):3-6.
ABSTRACT: The second leading cause of cancer in the United States, lung cancer kills more people than any other form of the disease. About 50% of patients have distant metastases at diagnosis, and most patients are diagnosed with advanced disease. Smoking is the most common risk factor for lung cancer, but smoking cessation reduces the risk (although gradually). Many drugs are available for the treatment of lung cancer. Chemotherapeutic regimens for lung cancer can be aggressive and complicated, and pharmacists can help patients understand what to expect and can recommend palliative therapy.
Lung cancer (LC) is the second leading cause of cancer in the United States.1 In 2011, an estimated 221,130 new cases were diagnosed (115,060 in men, 106,070 in women).2 LC is responsible for more cancer-related deaths than any other form of the disease, killing 1.2 million people per year worldwide.1-3 At diagnosis, about 50% of patients have distant metastases, and only 16% of patients have localized disease.4 The 5-year survival rate for LC is less than 15%.1 Most patients are diagnosed with advanced disease (stage IIIB or stage IV), which has a 5-year survival rate of less than 5%.5
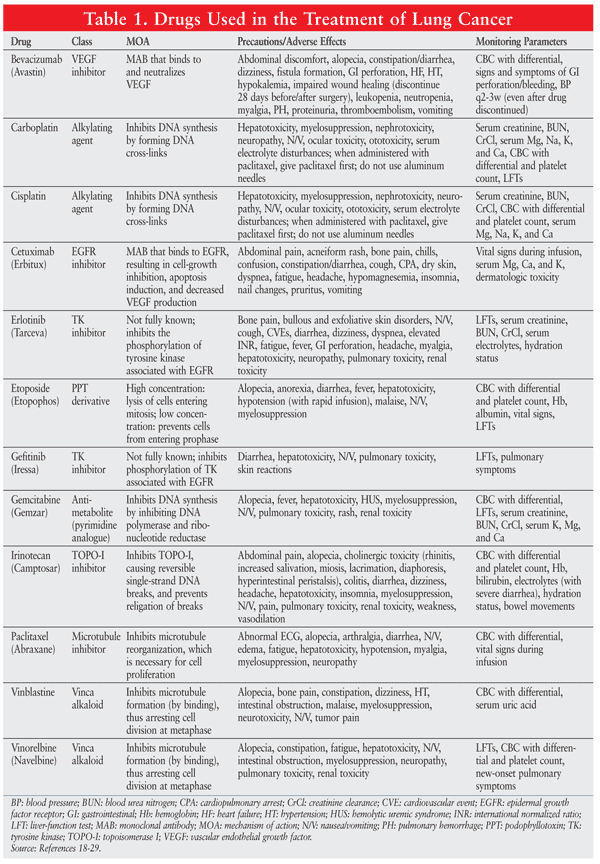
A variety of drugs are used in the treatment of LC. These agents are summarized in TABLE 1.
Risk Factors
LC may be one of the most preventable forms of cancer.6 Smoking, the most common risk factor for LC, is responsible for approximately 80% of all LC cases.4 Starting to smoke at an early age, a long duration of smoking, and a higher number of packs smoked daily are associated with an increased risk.5
Smoking cessation gradually reduces the risk of LC, but it takes at least 5 years to achieve a significant reduction. Even with smoking cessation, LC risk in former smokers will never equal that of never-smokers. Additionally, most experts believe that environmental tobacco smoke (secondhand smoke) increases LC risk by approximately 25%.4
Fewer than 20% of LCs are caused by other environmental respiratory carcinogens. Asbestos, benzene, arsenic, bis(chloromethyl) ether, hexavalent chromium, mustard gas, nickel, polycyclic aromatic hydrocarbons, and radon gas have been found to cause LC.1,4,6
Genetics may play a role in the development of LC. Risk is increased if a first-degree relative has had LC. Other factors are gender (women are more susceptible than men) and concomitant chronic obstructive pulmonary disease or asthma.1,4,5
Prior incidence of LC raises the risk of a second occurrence. Median survival in patients with a second occurrence is 1 to 2 years, with a 5-year survival rate of about 20%.5
Histology
More than 90% of lung neoplasms are one of four major cell types: adenocarcinoma, squamous cell, large cell, and small cell. Squamous cell, adenocarcinoma, and large cell have similar prognosis and treatment strategies and are collectively termed non–small cell lung cancer (NSCLC). About 10% of LC is associated with an overexpression of epidermal growth factor receptor (EGFR). This most commonly occurs in NSCLC and is a target for therapy.4
Squamous cell carcinoma, which occurs in the central portion of the lung, is highly associated with smoking.4,6 About half of LC cases are adenocarcinoma, which is the most common form in nonsmokers.4 Adenocarcinoma can present anywhere in the lung, but tends to occur in the periphery.6 Adenocarcinoma often metastasizes early in the disease process to distal sites such as the opposite lung, liver, bones, adrenal glands, kidneys, and central nervous system (CNS).4 Fewer than 10% of LC cases are large cell carcinoma, which tends to occur peripherally. Small cell lung cancer (SCLC) is highly associated with smoking and tends to occur centrally.6
More than one histologic cell type can be present either in the same lung or in each lung. This phenomenon, called synchronous tumors, worsens the prognosis.4,6
Presentation
The most common presenting signs and symptoms of LC are cough, dyspnea, and chest pain, with or without hemoptysis. Since many patients have concomitant respiratory disease (often caused by smoking), these symptoms are often ignored. If a tumor is in the periphery, it is likely to remain asymptomatic until it has grown or spread to other areas.4
Other symptoms depend upon the site(s) of any existing metastases. Presenting symptoms result from distant metastasis in approximately one-third of LC patients. The bones, liver, adrenal glands, brain, spinal cord, lymph nodes, and skin are the most common sites of metastasis.4,6
Regional thoracic metastasis can cause pleural effusion, pneumonitis, dysphagia (due to esophageal compression), pericardial effusion, cardiac tamponade, and tracheal obstruction. Weakness, anorexia, weight loss, hoarseness (due to laryngeal nerve paralysis), shoulder or arm pain, wheeze, stridor, and fever (sometimes) may be seen in some patients.4,6 Obstruction of the superior vena cava may present as distention of the veins in the neck and chest wall, cyanosis, facial plethora, and edema of the upper extremities.6
Liver metastasis is common at initial presentation in both NSCLC and SCLC. This rarely results in abnormal liver-function tests until the tumors become large and numerous. Weakness and weight loss are the most common symptoms of liver metastases.6
CNS symptoms may include headache, nausea, vomiting, seizures, confusion, and personality changes.6 Bone pain or fracture indicates metastasis to the bones.4 Paraneoplastic symptoms are not direct effects of the tumor, and they occur at sites distal from the primary tumor and any metastases. Most commonly seen in SCLC, these include Cushing’s syndrome, hypercalcemia (most often with squamous cell), inappropriate secretion of antidiuretic hormone, pulmonary hypertrophic osteoarthropathy (clubbing and inflammation of the long bones of the arms and legs), anemia, Eaton-Lambert myasthenic syndrome (autoimmune condition resulting in weakened muscles of the limbs), and hypercoagulation.4
EGFRs
Biomarkers can guide treatment by targeting certain biological processes. Mutations in EGFRs respond well (60%-90%) to the tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib. Approximately 10% of white patients have EGFR mutations. Unfortunately, a high percentage of patients who have a good response to TKIs later develop TKI-resistant disease because of a second mutation.6,7
NSCLC
Approximately 80% to 85% of LC cases are NSCLC.7 NSCLC grows more slowly than SCLC, but about 30% of patients present with stage III disease.3,4 First-line treatment for stage I/II NSCLC is lobectomy. Radiotherapy (RT) is an option for patients who are unable to undergo or refuse surgery.3,8
The European Society for Medical Oncology (ESMO) guidelines support the use of cisplatin-based adjuvant chemotherapy (CTX) in stage I/II LC.3 The American College of Chest Physicians (ACCP) guidelines are similar. The ACCP recommends adjuvant platinum-based CTX for stage II patients with good performance status; it does not recommend adjuvant CTX for stage IA/IB patients.8
There is not a preponderance of evidence on the use of neoadjuvant therapy before surgery, but it has advantages. Many more patients are compliant and undergo all three required treatments compared with adjuvant therapy, and clinical downstaging occurs in about 50% of patients. The ESMO does not advise against the use of neoadjuvant treatment. If neoadjuvant therapy is to be used in stage IIIA-N2 patients, the ESMO recommends three cycles of CTX with a two-drug combination consisting of cisplatin and a third-generation drug (vinorelbine, gemcitabine, taxanes, or irinotecan).3,9
For stage III patients, the ACCP advocates the use of adjuvant cisplatin-based CTX or RT after surgery. Chemoradiotherapy (CRT) is not recommended for resectable stage IIIA patients. If a patient is found to be stage IIIA prior to surgery, the ACCP recommends cisplatin-based CTX as primary treatment.10 For nonresectable stage III disease, concomitant CRT is the standard of care. The ESMO guidelines call for cisplatin-etoposide (vinblastine or vinorelbine may be substituted for etoposide). An alternative chemotherapeutic regimen is carboplatin-paclitaxel.3
First-line treatment of stage IV disease is a platinum-based agent administered with a third-generation drug. If platinum treatment is contraindicated, a combination of third-generation drugs may be used; however, this approach is less effective.7 Most patients should undergo three or four cycles of CTX. If the patient responds, a maximum of six cycles may be given.7,11 The American Society of Clinical Oncology (ASCO) recommends that, in stage IV LC, CTX be discontinued if the disease progresses or there is no response after four cycles.11
A single-agent CTX is recommended for elderly stage IV patients. In poorly performing patients, palliative treatment is indicated, rather than CTX. If these patients are found to have EGFR mutations, TKIs may be an option.7
Indications for RT in stage IV disease include pain due to neural compression, bone metastases, chest pain, cough or dyspnea, spinal cord compression, superior vena cava syndrome (obstruction of the superior vena cava), and bone fractures.7
Treatment options should be based on tumor histology, performance status, comorbidities, and patient preferences. Follow-up should include a CT scan every 6 months for 2 years, then annually.7
SCLC
Approximately 15% of all LC cases are SCLC, which is highly aggressive and grows rapidly. Sixty percent to 70% of patients with SCLC present with disseminated disease, with typical metastatic sites being the opposite lung, liver, adrenal glands and other endocrine organs, bones, and CNS.4 SCLC is strongly associated with smoking.12
SCLC is staged as limited-stage or extensive-stage disease. Limited-stage SCLC is treated with curative intent. The median survival of patients with limited-stage disease is 16 to 22 months.13
For limited-stage SCLC, current guidelines call for CTX in combination with RT. The recommended chemotherapeutic regimen is etoposide-cisplatin, which has a 5-year survival rate of 20% to 25%.12 There is debate surrounding the timing of RT in relation to CTX. Most studies show improved survival rates when RT is initiated concurrently with CTX in limited-stage disease.12
Extensive-stage SCLC is treated initially with CTX. The initial response rate is high (60%-70%); however, response wanes with continued treatment. Extensive-stage SCLC has a poor prognosis, with a median survival of 10 months and a 2-year survival rate of 10%.12,13 RT may be offered to patients with extensive-stage disease if there is a complete response to CTX outside the lungs and at least a partial response in the lungs, but evidence supporting improved survival is weak.13
The currently recommended CTX regimen is cisplatin (or carboplatin) plus etoposide (or irinotecan).12,13 Oral etoposide, although inferior, may be used in patients who cannot tolerate aggressive CTX.12 Four to six cycles of CTX are recommended for all stages of SCLC. There is no evidence of improved survival with maintenance therapy; therefore, maintenance is not recommended.12,13 Patients who relapse after their first CTX regimen may be offered a second-line regimen.13
Screening
Chest radiography, with and without sputum cytology, has been studied extensively. There is no evidence that chest radiography reduces mortality, so it is not recommended for LC screening.14
The National Lung Screening Trial (NLST) investigated low-dose helical CT as a screening tool for lung cancer. In the trial, low-dose CT resulted in reduced mortality. Compared with chest radiography, low-dose CT produced more positive results. NLST was the first large-scale randomized, controlled trial to demonstrate reduced LC mortality through use of a screening technique.15
A recent collaboration between the ACCP, ASCO, American Cancer Society, National Comprehensive Cancer Network, and American Thoracic Society resulted in new screening guidelines. These guidelines suggest that annual screening with low-dose CT be offered to patients aged 55 to 74 years who have smoked for at least 30 pack-years and either continue to smoke or have quit within the past 15 years. Screening should performed only in settings that can provide the same comprehensive care received by NLST participants.16 Patients who are younger than 55 years, have accumulated less than 30 pack-years, or quit more than 15 years ago should not be offered screening.16
Research is currently underway to detect LC biosignatures using a machine called a colorimetric sensor. The metabolic features of LC cells differ from those of normal cells. These metabolic differences result in the production of volatile organic compounds (VOCs) in LC. VOCs are carried through the blood and delivered to the lungs, where they are exhaled. To date, there has been moderate success with colorimetric sensors in detecting the presence of LC. When this machine is used with clinical predictors, accuracy is increased. This test could also distinguish between different histologic types and disease stages.17
Prevention
Smoking cessation is the most important behavioral modification for the prevention of LC, and it is strongly recommended for all LC patients because it improves outcomes and reduces adverse effects of therapy. However, former smokers remain at high risk for LC years after quitting, and more than 50% of LC cases occur in former smokers. Several agents have been studied to determine whether they offer any protection against LC.5
Based on epidemiologic data, consumption of at least three servings of fruits and vegetables per day is associated with a lower incidence of cancer; however, because beta-carotene increases the risk of LC, carrots should be avoided.5
Studies evaluating vitamin E, N-acetylcysteine, retinoids, and aspirin or nonsteroidal anti-inflammatory drugs have failed to show any protective effects against LC.5
To date, no agents have demonstrated chemopreventive properties in clinical trials. The ACCP does not recommend the use of any vitamins, supplements, or drugs for primary, secondary, or tertiary prevention of lung cancer.5
Conclusion
LC is the most deadly cancer, but it also may be one of the most preventable cancers. Smoking is the biggest risk factor, and smoking cessation is the cornerstone of prevention. The pharmacist has a duty to educate patients who smoke about LC and the poor prognosis. The pharmacist can also assist with smoking cessation.
Chemotherapeutic regimens for LC can be complicated and aggressive. The pharmacist is poised to be an educator to patients on what to expect and to help recommend palliative treatment.
REFERENCES
1. U.S. Preventive Services Task Force. Lung cancer screening:
recommendation statement.
www.uspreventiveservicestaskforce.org/3rduspstf/lungcancer/lungcanrs.htm.
Accessed April 2, 2012.
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the
impact of eliminating socioeconomic and racial disparities on premature
cancer deaths. CA Cancer J Clin. 2011;61:212-236.
3. Crino L, Weder W, van Meerbeeck J, Felip E. Early stage and
locally advanced (non-metastatic) non-small-cell-lung cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v103-v115.
4. Frieze DA, Adams VR. Lung cancer. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill Medical; 2011.
5. Gray J, Mao JT, Szabo E, et al. Lung cancer chemoprevention: AACP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl 3):56S-68S.
6. Johnson DH, Blot WJ, Carbone DP, et al. Cancer of the lung:
non-small cell lung cancer and small cell lung cancer. In: Abeloff MD,
Armitage JO, Niederhuber JE, et al, eds. Abeloff’s Clinical Oncology. 4th ed. Philadelphia, PA: Churchill Livingstone; 2008.
7. D’Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 2010;21(suppl 5):v116-v119.
8. Scott WJ, Howington J, Feigenberg S, et al. Treatment of non-small
cell lung cancer stage I and stage II: ACCP evidence-based clinical
practice guidelines (2nd edition). Chest. 2007;132(suppl 3):234S-242S.
9. Chen Y, Okunieff P. Radiation and third-generation chemotherapy. Hematol Oncol Clin North Am. 2004;18:55-80.
10. Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW. Treatment
of non-small cell lung cancer—stage IIIA: ACCP evidence-based clinical
practice guidelines (2nd edition). Chest. 2007;132(suppl 3):243S-265S.
11. Azzoli CG, Temin S, Aliff T, Baker S Jr, et al. 2011 focused
update of 2009 American Society of Clinical Oncology clinical practice
guideline update on chemotherapy for stage IV non-small-cell lung
cancer. J Clin Oncol. 2011;29:3825-3831.
12. Sorensen M, Pijls-Johannesma M, Felip E. Small-cell lung cancer:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 2010;21(suppl 5):v120-v125.
13. Simon GR, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(suppl 3):324S-339S.
14. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest
radiograph and lung cancer mortality: the Prostate, Lung, Colorectal,
and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873.
15. The National Lung Screening Trial Research Team. Reduced
lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
16. Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:E1-E12.
17. Mazzone PJ, Wang XF, Xu Y, Mekhail T, et al. Exhaled breath
analysis with a colorimetric sensor array for the identification and
characterization of lung cancer. J Thorac Oncol. 2012;7:137-142.
18. Cisplatin product information. Bedford, OH: Bedford Laboratories; November 2011.
19. Carboplatin product information. Bedford, OH: Bedford Laboratories; November 2009.
20. Navelbine (vinorelbine tartrate) product information. Research Triangle Park, NC: GlaxoSmithKline; November 2002.
21. Vinblastine sulfate product information. Bedford, OH: Bedford Laboratories; April 2010.
22. Gemzar (gemcitabine) product information. Indianapolis, IN: Eli Lilly and Co; February 2011.
23. Abraxane (paclitaxel) product information. Summit, NJ: Abraxis Bioscience, LLC; January 2012.
24. Camptosar (irinotecan) product information. New York, NY: Pfizer; November 2009.
25. Etopophos (etoposide) product information. Princeton, NJ: Bristol-Myers Squibb; March 2011.
26. Tarceva (erlotinib) product information. Farmingdale, NY: OSI Pharmaceuticals, LLC; April 2012.
27. Erbitux (cetuximab) product information. Branchburg, NJ: ImClone LLC; January 2012.
28. Avastin (bevacizumab) product information. South San Francisco, CA: Genentech, Inc; May 2012.
29. Iressa (gefitinib) product information. Wilmington, DE: AstraZeneca; June 2005.
To comment on this article, contact rdavidson@uspharmacist.com.





