US Pharm. 2013;38(5)(Oncology suppl):8-11.
ABSTRACT: Prostate cancer is the most common cancer in men (excluding nonmelanoma skin cancer) and the second most common cause of death from cancer among white, African American, American Indian/Native Alaskan, and Hispanic men. The epidemiology of prostate cancer is not fully understood; however, several risk factors have been documented, including increasing age, family history, and race. Vitamin D also has been suggested as a risk factor. Dietary vitamin D intake, serum vitamin D levels, and sunlight exposure have been investigated to determine whether vitamin D is associated with an increased or decreased risk of prostate cancer. Epidemiologic studies evaluating the correlation between risk of prostate cancer and vitamin D levels are conflicting.
Excluding nonmelanoma skin cancer, prostate cancer is the most common cancer in men, regardless of race or ethnicity.1 It is the second most common cause of death from cancer among white, African American, American Indian/Native Alaskan, and Hispanic men. In 2008, a total of 214,633 men were diagnosed with prostate cancer in the United States, and 28,471 men died from the disease.
As with many other cancers, the epidemiology of prostate cancer is not fully understood. Several risk factors have been documented, including increasing age, family history, and race.1,2 African American men have a higher risk of prostate cancer compared with Caucasian men. Environmental factors (latitude and diet), inflammation, and obesity have been shown to be risk factors for prostate cancer, but their precise role is unclear.3 Vitamin D has been suggested as a risk factor for prostate cancer.2-4 Several documented risk factors—dark skin, diet, and latitude—have been linked to vitamin D availability.
VITAMIN D
Vitamin D is a fat-soluble steroid prohormone that is available from foods (either naturally or through fortification) and vitamins or supplements.3 Vitamin D3 is also produced from skin exposure to ultraviolet B rays (UVB). Vitamins D2 (synthesized by plants) and D3 are metabolized to 25(OH)D, also known as calcidiol, in the liver and then metabolized to 1,25(OH)2D, or calcitriol, in the kidneys and other target tissues, such as the colon and prostate.3,5,6 Circulating 25(OH)D is thought to be the best indicator of vitamin D status, since it reflects exogenous vitamin D sources, endogenous production, and the actions of metabolic enzymes.3,4,6,7
Vitamin D receptors (VDRs) are present in both cancerous and noncancerous prostate cells.3,4,6,8 VDRs mediate the antiproliferative action of 1,25(OH)2D.2,3 They also have been shown to induce apoptosis, cause differentiations, inhibit telomerase expression, prevent tumor cell invasiveness, and suppress tumor-induced angiogenesis in vitro and in vivo.2,3,9 Epidemiologic studies evaluating the correlation between vitamin D levels and prostate cancer risk are conflicting.3,6 Vitamin D has been associated with both an increased and a decreased risk of prostate cancer.
EPIDEMIOLOGIC STUDIES
Dietary Vitamin D and Risk of Prostate Cancer
The association between dietary vitamin D intake and prostate cancer risk has been examined in several epidemiologic studies.3 Results of these studies are negative or conflicting; however, limited data indicate a possible link. One study concluded that men in the U.S. have a 10-fold greater risk of developing prostate cancer compared with men in Japan.10 The increased risk has been linked to the difference in diet between American and Japanese men. Japanese men have a higher consumption of fatty fish, and thus an increased amount of vitamin D and omega-3 fatty acids. Omega-3 fatty acids dissociate vitamin D metabolites from binding proteins, thereby increasing active levels of these metabolites in the blood.3 In another study, Ahn et al noted a reduced risk of prostate cancer with greater intake of supplemental vitamin D.11 A 40% risk reduction occurred in patients receiving more than 600 IU of supplemental vitamin D versus those not receiving it. However, dietary intake of vitamin D (i.e., not via supplements) was not associated with a decreased or increased risk of prostate cancer.11
The lack of association between dietary vitamin D and prostate cancer risk has been documented. The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study was designed to test the effects of alpha-tocopherol and beta-carotene supplements on cancer incidence and mortality.12 This primary prevention trial also examined associations between multiple nutrients believed to impact 1,25(OH)2D and prostate cancer risk. Vitamin D was not associated with prostate cancer risk, and this lack of association has been noted in other epidemiologic studies.12-18
Serum Vitamin D and Prostate Cancer Risk
The association between serum vitamin D levels and prostate cancer has been investigated in multiple observational studies. TABLE 1 summarizes these studies in detail.1,2,4,7,11,19-26
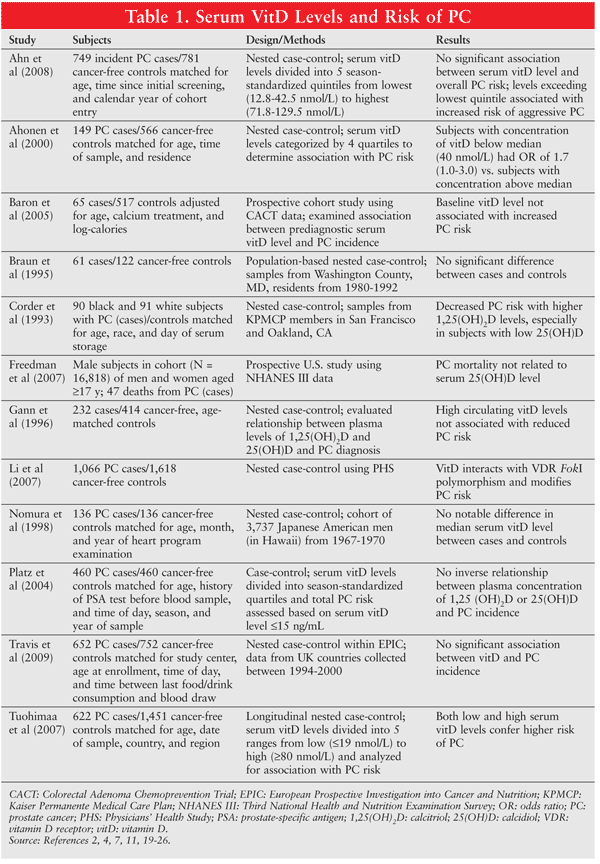
Schwartz and Hulka proposed protective effects of vitamin D against prostate cancer, noting that prostate cancer incidence increased with age and vitamin D levels decreased with age.27 Additionally, mortality rates from prostate cancer in the U.S.were inversely correlated with UVB, a primary source of vitamin D.
Ahonen et al conducted a nested case-control study within a cohort of 18,966 men aged 40 to 55 years from the Helsinki Heart Study.4 Low 25(OH)D levels were associated with an increased risk of prostate cancer.4 In a case-control study, Li et al evaluated a large number of prostate cancer patients and cancer-free controls from the Physician’s Health Study.7 A significantly increased risk of aggressive prostate cancer (odds ratio = 2.1, 95% CI = 1.2-3.4) was observed in subjects whose 25(OH)D and 1,25(OH)2D levels were below the median. The median plasma level of 25(OH)D was 25 ng/mL for winter-spring collection and 32 ng/mL for summer-autumn collection. The median plasma level of 1,25(OH)2D was 32 ng/mL and did not differ according to collection period.7
Several studies failed to find an association between serum 25(OH)D and prostate cancer, and the hypothesis that high serum levels of vitamin D are associated with a reduced incidence of prostate cancer is not supported.11,23-25 A nested case-control study by Ahn et al evaluated the association of serum 25(OH)D and prostate cancer risk in participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.11 No statistically significant trend in overall prostate cancer risk with increasing serum 25(OH)D level was found.11 In a large cohort study of Japanese American men that was conducted by Nomura et al, 136 confirmed cases of prostate cancer were noted.24 A statistically significant association between serum vitamin D and prostate cancer was not observed; however, the blood samples for vitamin D levels were drawn 23 years prior, and not necessarily at the time of cancer diagnosis. It is likely that changes in vitamin D levels occurred over this time period.3
Tuohimaa et al performed a nested case-control study of Nordic men from the Helsinki Heart Study and the Northern Sweden Health and Disease Cohort.2 Prostate cancer and serum vitamin D levels were analyzed using quintiles. In the overall study population, both low (<19 nmol/L) and high (>80 nmol/L) concentrations of 25(OH)D were associated with an increased risk of prostate cancer.2
Sunlight and Prostate Cancer Risk
Since UVB promotes the synthesis of vitamin D3, sunlight may indirectly affect the risk of prostate cancer. Multiple studies have been conducted to evaluate the association between sun exposure and prostate cancer risk.
Hanchette and Schwartz analyzed death secondary to prostate cancer in relation to sunlight exposure and found a negative correlation (P <.0001); i.e., patients exposed to more sunlight were less likely to die from prostate cancer.10 A case-control study (450 cases, 455 controls) by John et al revealed an important role for sun exposure and VDR polymorphisms in the etiology of prostate cancer; a reduced risk of prostate cancer was observed with high sun exposure and high occupational outdoor exposure.28 Additionally, in a cohort study by de Vries et al, skin cancer patients had a reduced risk of developing prostate cancer compared with the general population (standardized incidence ratio = 0.89, 95% CI = 0.78, 0.99), particularly shortly after diagnosis.29 Several studies suggest that host factors may affect UVB-induced vitamin D synthesis and that polymorphisms that mediate vitamin D effectiveness may determine the association between sunlight exposure and prostate cancer risk.3,30-33
CONCLUSION
Overall, the data regarding an association between serum levels of vitamin D and prostate cancer are conflicting. A report by the International Agency for Research on Cancer stated that evidence linking vitamin D and prostate cancer was lacking.34
Dietary intake does not appear to be protective against prostate cancer; however, as noted earlier, the study by Ahn et al found a 40% risk reduction in patients receiving more than 600 IU of supplemental vitamin D versus those not taking vitamin D supplements.11 Studies investigating the association of prostate cancer and serum vitamin D levels had the most variation in results.
Conflicting evidence may be due to study limitations. In Ahonen et al, blood samples were obtained in the early 1980s and used for analysis once a subject had a confirmed case of prostate cancer.4 Tuohimaa et al used serum samples from the Janus Project, which was initiated in 1973; follow-up continued until 1997 (24 years).2 During this time frame, fluctuation in vitamin D levels would be inevitable, but such changes are not reflected in most studies because the samples were drawn only at baseline.3 Most research has involved observational nested case-control studies within larger cohorts. Finally, many studies, including those by Ahonen et al and Tuohimaa et al, used data from cohorts that were assembled to assess cardiovascular disease.2,4
Given that epidemiologic studies are conflicting, further studies are warranted to assess the possible association between vitamin D and prostate cancer.
REFERENCES
1. CDC. Prostate cancer. www.cdc.gov/cancer/prostate. Accessed October 26, 2012.
2. Tuohimaa P, Pukkala E, Scélo G, et al. Does solar exposure, as
indicated by the non-melanoma skin cancers, protect from solid cancers:
vitamin D as a possible explanation. Eur J Cancer. 2007;43:1701-1712.
3. Gupta D, Lammersfeld CA, Trukova K, Lis CG. Vitamin D and prostate cancer risk: a review of the epidemiological literature. Prostate Cancer Prostatic Dis. 2009;12:215-226.
4. Ahonen MH, Tenkanen L, Teppo L, et al. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11:847-852.
5. Plaut D, McLellan W. Vitamin D. J Continuing Educ Topics Issues. 2011;13:6-9.
6. Schwartz GG. Vitamin D and the epidemiology of prostate cancer. Semin Dial. 2005;18:276-289.
7. Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma
vitamin D metabolites, vitamin D receptor polymorphisms, and prostate
cancer. PLoS Med. 2007;4:e103.
8. Vijayakumar S, Mehta RR, Boerner PS, et al. Clinical trials involving vitamin D analogs in prostate cancer. Cancer J. 2005;11:362-373.
9. Miller GJ, Stapleton GE, Ferrara JA, et al. The human prostatic
carcinoma cell line LNCaP expresses biologically active, specific
receptors for 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1992;52:515-520.
10. Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer
mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861-2869.
11. Ahn J, Peters U, Albanes D, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796-804.
12. ATBC Cancer Prevention Study Group. The Alpha-Tocopherol,
Beta-Carotene Lung Cancer Prevention Study: design, methods, participant
characteristics, and compliance. Ann Epidemiol. 1994;4:1-10.
13. Chan JM, Pietinen P, Virtanen M, et al. Diet and prostate cancer
risk in a cohort of smokers, with a specific focus on calcium and
phosphorus (Finland). Cancer Causes Control. 2000;11:859-867.
14. Tavani A, Bertuccio P, Bosetti C, et al. Dietary intake of calcium, vitamin D, phosphorus and the risk of prostate cancer. Eur Urol. 2005;48:27-33.
15. Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and
vitamin D intakes and prostate cancer risk in the National Health and
Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr. 2005;81:
1147-1154.
16. Park SY, Murphy SP, Wilkens LR, et al. Calcium, vitamin D, and
dairy product intake and prostate cancer risk: the Multiethnic Cohort
Study. Am J Epidemiol. 2007;166:1259-1269.
17. Berndt SI, Carter HB, Landis PK, et al. Calcium intake and
prostate cancer risk in a long-term aging study: the Baltimore
Longitudinal Study of Aging. Urology. 2002;60:1118-1123.
18. Kristal AR, Cohen JH, Qu P, Stanford JL. Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:719-725.
19. Baron JA, Beach M, Wallace K, et al. Risk of prostate cancer in a randomized clinical trial of calcium supplementation. Cancer Epidemiol Biomarkers Prev. 2005;14:586-589.
20. Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Prostate cancer
and prediagnostic levels of serum vitamin D metabolites (Maryland,
United States). Cancer Causes Control. 1995;6:235-239.
21. Corder EH, Guess HA, Hulka BS, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev. 1993;2:467-472.
22. Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study
of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594-1602.
23. Gann PH, Ma J, Hennekens CH, et al. Circulating vitamin D
metabolites in relation to subsequent development of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:121-126.
24. Nomura AM, Stemmermann GN, Lee J, et al. Serum vitamin D
metabolite levels and the subsequent development of prostate cancer
(Hawaii, United States). Cancer Causes Control. 1998;9:425-432.
25. Platz EA, Leitzmann MF, Hollis BW, et al. Plasma 1,25-dihydroxy-
and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255-265.
26. Travis RC, Crowe FL, Allen NE, et al. Serum vitamin D and risk of
prostate cancer in a case-control analysis nested within the European
Prospective Investigation into Cancer and Nutrition (EPIC). Am J Epidemiol. 2009;169:1223-1232.
27. Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res. 1990;10:1307-1311.
28. John EM, Schwartz GG, Koo J, et al. Sun exposure, vitamin D
receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470-5479.
29. de Vries E, Soerjomataram I, Houterman S, et al. Decreased risk
of prostate cancer after skin cancer diagnosis: a protective role of
ultraviolet radiation? Am J Epidemiol. 2007;165:966-972.
30. Luscombe CJ, Fryer AA, French ME, et al. Exposure to ultraviolet
radiation: association with susceptibility and age at presentation with
prostate cancer. Lancet. 2001;358:641-642.
31. Moon SJ, Fryer AA, Strange RC. Ultraviolet radiation: effects on risks of prostate cancer and other internal cancers. Mutat Res. 2005;571:207-219.
32. Bodiwala D, Luscombe CJ, Liu S, et al. Prostate cancer risk and
exposure to ultraviolet radiation: further support for the protective
effect of sunlight. Cancer Lett. 2003;192:145-149.
33. Rukin NJ, Zeegers MP, Ramachandran S, et al. A comparison of
sunlight exposure in men with prostate cancer and basal cell carcinoma. Br J Cancer. 2007;96:523-528.
34. International Agency for Research on Cancer (IARC). Vitamin D and Cancer. IARC Working Group Reports, Vol. 5. Lyon, France: IARC; 2008.
To comment on this article, contact rdavidson@uspharmacist.com.





