US Pharm. 2013;38(9)(Oncology suppl):3-7.
ABSTRACT: Inflammatory breast cancer (IBC) is an uncommon and highly aggressive disease that is difficult to diagnose. IBC is frequently misdiagnosed and treated as mastitis, leading to tumor progression prior to detection. Disease progresses rapidly—in a matter of weeks to months—and generally is stage III or IV upon diagnosis. IBC is characterized by the appearance of inflammation in one breast and typically involves pain and fast-progressing breast tenderness, firmness, and enlargement. IBC accounts for 1% to 5% of all breast cancers diagnosed in the United States. Although diagnosis is challenging, it is important that all women be screened regularly to identify this cancer at an earlier stage and improve survival.
Inflammatory breast cancer (IBC), first described by Lee and Tannenbaum,1 is the most vicious manifestation of breast cancer. Although IBC incidence in the United States is relatively low (1% to 5%),2 it appears to be increasing. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, the IBC incidence rate per 100,000 woman-years increased from 2.0 in 1988–1990 to 2.5 (P <.001) in 1997–1999, whereas the non-IBC rate decreased from 108 to 101 (P = .0084).3 This article presents a review of IBC that includes a real case scenario of a patient with IBC.
Presentation
The clinical presentation of IBC is unique, with distinct characteristics. Haagensen first described the classic criteria of IBC: edema over at least two-thirds of the breast, peau d’orange (pitting, like an orange rind), diffuse erythema, tenderness, induration, warmth, enlargement, and tumor diffuseness upon palpation.4 Consistent with Haagensen’s original description, the gold standard definition of IBC developed by the American Joint Committee on Cancer considers IBC a clinicopathological entity characterized by diffuse erythema and edema, frequently without an underlying palpable mass.5 Unfortunately, diagnostic criteria for IBC are nonspecific, and patients are often misdiagnosed with mastitis. Misdiagnosis of IBC leads to a delay in management.
REAL CASE SCENARIO: PRESENTATION (June 2011)
Chief complaint: RR, a 51-year-old Hispanic female with history of hypertension, visits emergency department and reports having right breast pain for about 1 month. RR visited Planned Parenthood yesterday and was diagnosed with cellulitis (given prescription for dicloxacillin; not filled). Reports intermittent chill. Last mammogram approximately 1 year ago—within normal limits.
Breast cancer risk history: menarche age 14, 2-year history of oral contraceptives, perimenopausal, pregnancies 2/preterm 1/abortions 1/living children 1, 1st full pregnancy age 29, breastfeeding—yes.
Family history: grandmother—diabetes; brother—colitis; brother—schizophrenia; sister—thyroid issues; sister—multiple sclerosis; father (deceased)—gastric cancer; mother—stroke age 60s.
Substance-use history: no smoking/alcohol/drugs.
Positive risk factors for developing breast cancer: oral contraceptive use. Negative risk factors for developing breast cancer: family history, Hispanic ethnicity, menarche age 14, pregnancy age 29, breastfeeding.
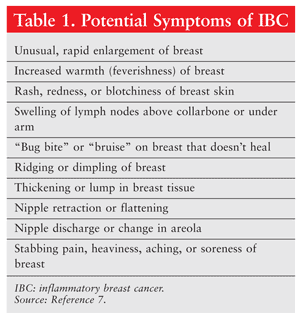
IBC presentation typically involves a tender, firm, painfully enlarged breast. The skin of the breast is thickened and warm, and the color ranges from pink to a purplish hue similar to ecchymosis.6 The breast may appear pitted (peau d’orange), and the skin may be ridged. If the nipple is involved, it may be flattened, crusted, blistered, red, or retracted. Symptoms (TABLE 1)7 progress rapidly, and patients often have axillary node involvement by the time they seek medical attention; approximately one-third will have distant metastases.8
The growth pattern of IBC is minimally in situ compared with non-IBC, leaving more available space for migration of cancer cells. With the vast migration of cells, lymph vessels become blocked, preventing normal flow through the tissue. This process, known as dermal lymphatic invasion (DLI),9,10 is a hallmark of IBC and is ultimately responsible for its highly metastatic potential.11
IBC tends to be highly angiogenic,12,13 and numerous experimental studies have identified potential pathogenetic markers that may be key in the development and progression of this disease. Nine angiogenic factors have been quantified via reverse transcription polymerase chain reaction: vascular endothelial growth factor (VEGF), VEGFR1, VEGFR2, angiopoietin (Ang)1, Ang2, tyrosine kinase with immunoglobulinlike and EGF-like domains (TIE) 1, TIE2, cyclo-oxygenase 2, and basic fibroblast growth factor (bFGF). Of these, Ang1, TIE1, TIE2, and bFGF are strongly expressed in IBC versus non-IBC.14
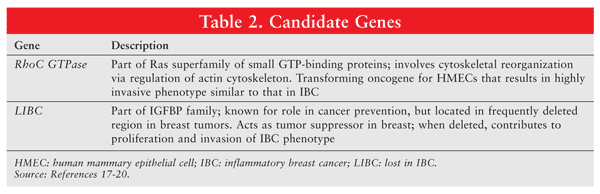
Pathogenetic markers expressed in IBC have been identified, with certain cell lines established (SUM149, SUM190).15 A landmark study by Lerebours et al confirmed frequent deletions and gains in 66 human IBC samples.16 The 52% overall rate of loss of heterozygosity at 21 chromosomal regions on 12 chromosomal arms associated with primary breast cancer indicated high genomic instability in IBC cells.16 van Golen et al compared expression of transcripts from one cell line (SUM149) actively growing normal mammary epithelial cells with patients’ matched lymphocytes and identified 17 differentially expressed genes.17 Of these 17 genes, RhoC GTPase and LIBC (lost in inflammatory breast cancer) (TABLE 2) were the only ones overexpressed in IBC samples and underexpressed in stage III non-IBC samples. RhoC was overexpressed in 90% of IBC samples versus 36% of non-IBC samples; LIBC was underexpressed in 80% versus 21%, respectively.18-20 RhoC GTPase expression appears to be modified by LIBC, and these genes might act in concert to cause IBC.21
Many experimental studies have brought to light other pathogenetic factors involved in IBC progression, such as hormone receptor (HR) status, the p53 tumor-suppressor gene, chemokine receptors, and cytokines.8 There seems to be a higher frequency of negative estrogen receptor (ER) and progesterone receptor (PR) status in IBC tumors, with some studies reporting up to 83% of tumors being ER-negative.22 Breast cancers lacking ER and PR expression are associated with a worse prognosis and shorter overall survival (OS).23
Guérin et al noted that although human epidermal growth factor 2 (HER2) is not specific for IBC, about 60% of IBCs overexpress HER2.24 This particular molecular marker may denote patients with a worse prognosis. However, in a case-only analysis of more than 2,000 patients with IBC, Dawood et al10 found that breast cancer–specific survival favoring patients with HER2-positive IBC tumors versus those with HER2-negative tumors had only borderline significance (hazard ratio 0.82, 95% CI 0.68-0.99).25 The role of HER2 in IBC remains to be defined.
p53 mutations, which occur in 20% to 50% of human breast cancers,26 are associated with poor OS.27 Chemokine receptors CXCR4 and CCR7 expressed in IBC tumors also have been linked to poor OS.28 As for cytokines, IBCs tend to produce few inflammatory markers such as interferon gamma, interleukin (IL)-1, and IL-12. On the other hand, IBC tumor cell lines do release VEGF, bFGF, IL-6, and IL-8; these cytokines are involved in RhoC GTPase overexpression, which is specifically associated with IBC.29
Differential Diagnosis
IBC can be difficult to diagnose since there is no lump to be detected during a routine examination or by mammography. IBC is aggressive; it can develop between scheduled mammograms and progress rapidly. Conditions such as mastitis and breast abscess may be confused with IBC, resulting in delayed diagnosis.
To accurately diagnose IBC, a core biopsy guided by palpation, sonography, or stereotactic radiography should be performed. Since a hallmark of IBC is DLI, a full-thickness biopsy should be obtained. Additionally, all invasive breast cancers should undergo testing for HRs and HER2. An international expert group on IBC advises the performance of at least two skin-punch biopsies in every patient with suspected IBC.10
REAL CASE SCENARIO: DIAGNOSIS (August 2011)
Chief complaint: RR complains of headache, cramps, and nausea.
History of present illness: 52-year-old Hispanic female visits clinic for follow-up on diagnosis of breast cancer. RR previously visited emergency department in 6/2011 with locally advanced inflammatory breast cancer (IBC). Initial bone scan positive for metastases.
Diagnosis: estrogen receptor–positive/progesterone receptor–positive/human epidermal growth factor 2/Neu-positive IBC.
RR, having been misdiagnosed with cellulitis initially, has developed metastatic IBC, an unfortunate path when diagnosis, treatment, and/or management is delayed.
Treatment
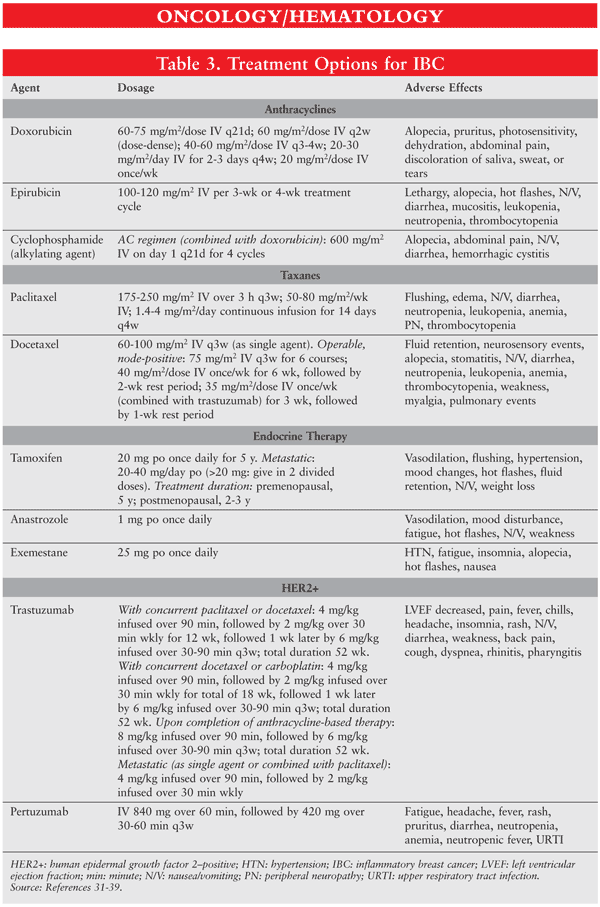
Standard treatment is an optimal sequence of therapy (FIGURE 1) taking a multimodal approach (TABLE 3): neoadjuvant chemotherapy followed by locoregional therapy.30-38 Surgery should not be attempted initially, since it is probable that residual disease will be left behind. Anthracyclines and taxanes are the most commonly recommended agents, since they are predominantly effective in IBC,39 but the best regimen has yet to be defined. Following neoadjuvant chemotherapy, mastectomy and radiotherapy should be performed to rid the area of residual disease.

In Dawood et al, the expert panel strongly recommended trastuzumab administration in patients with HER2-positive disease, based on studies indicating that its addition to primary chemotherapy was associated with improved pathological complete response rates.10 In addition, patients with HR-positive IBC should receive at least 5 years of hormone therapy with tamoxifen (premenopausal or postmenopausal patients) and/or an aromatase inhibitor (postmenopausal patients only).
REAL CASE SCENARIO: TREATMENT (November 2012)
Dose-dense doxorubicin and cyclophosphamide (dd-AC) started 6/2011.
Status post cycle 4 of dd-AC, paclitaxel and trastuzumab added to regimen 11/2011. Completed 1 year of trastuzumab.
Despite adequate treatment, breast cancer progression with cerebellar metastases diagnosed 11/2012.
Begin lapatinib (Tykerb) 1,000 mg daily (21-day regimen). Continue paclitaxel.
RR began treatment with the most commonly recommended classes of anticancer drugs: anthracyclines and taxanes. After the dd cycle, trastuzumab was added to RR’s regimen based on human epidermal growth factor 2 (HER2)/Neu-positive status. Unfortunately, the cancer metastasized, and lapatinib was added to the regimen to curb metastatic progression. Lapatinib is used in HER2+ advanced disease after previous unsuccessful treatments with other agents (anthracyclines, taxanes, and trastuzumab).
Follow-Up
Guidelines set forth by the American Society of Clinical Oncology in 2006 recommend that all breast cancer patients receive a physical examination every 3 to 6 months for the first 3 years, every 6 to 12 months for years 4 and 5, and annually thereafter.40 The physical examination should be done in combination with a mammogram of the contralateral breast annually.
REAL CASE SCENARIO: FULL REPORT
Chief complaint: RR complains of HA, cramps, and nausea.
History of present illness: 52-year-old Hispanic female visits clinic for follow-up on diagnosis of breast cancer. RR previously visited emergency department (ED) in 6/2011 with locally advanced inflammatory breast cancer (IBC). Initial bone scan positive for metastases.
ED note—chief complaint: RR, a 51-year-old Hispanic female with history of hypertension, reports having right breast pain for about 1 month. RR visited Planned Parenthood yesterday and was diagnosed with cellulitis (given prescription for dicloxacillin; not filled). Reports intermittent chill. Last mammogram approximately 1 year ago—within normal limits.
Past medical history: right IBC (stage IV) diagnosed 6/2011, prior breast biopsy 5/2003 complicated by hematoma; uterine fibroids with past endometrial polypectomy; carpal tunnel syndrome; tendinitis; sciatica; allergic rhinitis; hypertension; hemorrhoids.
Breast cancer risk history: menarche age 14, 2-year history of oral contraceptives, perimenopausal, pregnancies 2/preterm 1/abortions 1/living children 1, 1st full pregnancy age 29, breastfeeding—yes.
Family history: grandmother—diabetes; brother—colitis; brother—schizophrenia; sister—thyroid issues; sister—multiple sclerosis; father (deceased)—gastric cancer; mother—stroke age 60s.
Substance-use history: no smoking/alcohol/drugs.
Allergies: codeine (gastrointestinal upset), Vicodin (dizziness).
Vital statistics: height 159 cm; weight 85.7 kg; body-mass index 33.9; temperature (°F) 97.4; pulse 87; blood pressure 105/59; oxygen saturation 98%. Pain scale 6/10.
Examination: General appearance—pleasant. Eastern Cooperative Oncology Group: 1. Breast: right breast status postmastectomy—significant skin peeling and open areas related to radiation, left breast normal. Echocardiogram 3/2012: normal left ventricular size and systolic function with ejection fraction 60%-65%; no significant abnormalities noted. Pathology: right breast mastectomy with axillary dissection; infiltrating carcinoma with lobular and ductal features—grade 3 (poorly differentiated); vascular invasion present; metastatic carcinoma identified in 6 of 9 lymph nodes; size of largest metastasis 0.4 cm. Bone scan 8/12/2012: findings highly typical of widespread bony metastatic disease; mild uptake in C2/C3; uptake in T7, L4, multiple ribs bilaterally, left 6th costovertebral joint, left anterior superior iliac spine, and right ischial tuberosity.
Diagnosis: estrogen receptor–positive/progesterone receptor–positive/human epidermal growth factor 2/Neu-positive IBC.
Treatment: dose-dense doxorubicin and cyclophosphamide (dd-AC) started 9/2011. Status post cycle 4 of dd-AC, paclitaxel and trastuzumab added to regimen 11/2011. Completed 1 year of trastuzumab. Despite adequate treatment, breast cancer progression with cerebellar metastases diagnosed 11/2012. Begin lapatinib (Tykerb) 1,000 mg daily (21-day regimen); continue paclitaxel.
Survival Rates
According to the American Cancer Society, IBC is an aggressive cancer because of its rapid progression, its tendency to have spread by the time it is detected, and its likelihood of recurrence after treatment compared with most other types of breast cancer.41 Thus, IBC generally has a poor prognosis. In the past, IBC patients survived only about an average of 18 months after diagnosis. However, survival has improved with recent treatment advances, such as the combination of chemotherapy, radiation, and surgery.
According to SEER data, the 5-year relative rate of survival in patients with IBC versus those with non-IBC is about 40% (compared with 87% for all breast cancers combined).41
Pharmacist’s Role
The pharmacist’s role in the treatment of patients with IBC is significant. Treatments for this disease carry a significant risk of potentially serious toxicities, such as neuropathy, neutropenia, nausea, vomiting, and pain. The pharmacist can frequently assess IBC patients for signs of toxicity, as well as recommend methods for helping patients remain adherent to their treatment regimen.
REFERENCES
1. Lee B, Tannenbaum E. Inflammatory carcinoma of the breast: a report of twenty-eight cases from the breast clinic of Memorial Hospital. Surg Gynecol Obstet. 1924;39:580-595.
2. Maalej M, Frikha H, Ben Salem S, et al. Breast cancer in Tunisia: clinical and epidemiological study [article in French]. Bull Cancer. 1999;86:302-306.
3. Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97(13):966-975.
4. Haagensen CD. Inflammatory carcinoma. In: Haagensen CD, ed. Diseases of the Breast. 2nd ed. Philadelphia, PA: Saunders; 1971:576-584.
5. Breast. In: Green FL, Page DL, Fleming ID, et al, eds. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag; 2003:255-281.
6. Walshe JM, Swain SM. Clinical aspects of inflammatory breast cancer. Breast Dis. 2005-2006;22:35-44.
7. National Comprehensive Cancer Network. Understanding inflammatory breast cancer. www.nccn.com/understanding-cancer/166-understanding-inflammatory-breast-cancer.html. Accessed August 5, 2013.
8. Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423-429.
9. Bonnier P, Charpin C, Lejeune C, et al. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62:382-385.
10. Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515-523.
11. Jardines L, Haffty B, Theriault R. Locally advanced, locally recurrent and metastatic breast cancer. In: Pazdur R, Coia LR, Hoskins WJ, Wagman LD, eds. Cancer Management: A Multidisciplinary Approach. 3rd ed. Melville, NY: PRR; 1999:73-88.
12. Lerebours F, Bieche I, Lidereau R. Update on inflammatory breast cancer. Breast Cancer Res. 2005;7:52-58.
13. Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br J Cancer. 2003;88:718-725.
14. Van der Auwera I, Benoy I, Elst H, et al. Quantitative study of the angiogenic profile of inflammatory and noninflammatory breast cancer using real-time RT-PCR [abstract]. Breast Cancer Res Treat. 2003;82:s135.
15. Forozan F, Veldman R, Ammerman CA, et al. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br J Cancer. 1999;81:1328-1334.
16. Lerebours F, Bertheau P, Bieche I, et al. Evidence of chromosome regions and gene involvement in inflammatory breast cancer. Int J Cancer. 2002;102:618-622.
17. van Golen KL, Davies S, Wu ZF, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511-2519.
18. Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509-514.
19. van Golen KL, Wu ZF, Qiao XT, et al. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832-5838.
20. Kleer CG, Zhang Y, Pan Q, et al. WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene. 2002;21:3172-3180.
21. Kleer CG, Zhang Y, Pan Q, et al. WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res. 2004;6:R110-R115.
22. Harvey HA, Lipton A, Lawrence BV, et al. Estrogen receptor status in inflammatory breast carcinoma. J Surg Oncol. 1982;21:42-44.
23. Paradiso A, Tommasi S, Brandi M, et al. Cell kinetics and hormonal receptor status in inflammatory breast carcinoma. Comparison with locally advanced disease. Cancer. 1989;64:1922-1927.
24. Guérin M, Sheng ZM, Andrieu N, Riou G. Strong association between c-myb and oestrogen-receptor expression in human breast cancer. Oncogene. 1990;5:131-135.
25. Zell JA, Tsang WY, Taylor TH, et al. Prognostic impact of human epidermal growth factor-like receptor 2 and hormone receptor status in inflammatory breast cancer (IBC): analysis of 2,014 IBC patient cases from the California Cancer Registry. Breast Cancer Res. 2009;11:R9.
26. Moll UM, Riou G, Levine AJ. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci U S A. 1992;89(15):7262-7266.
27. Gonzalez-Angulo AM, Sneige N, Buzdar AU, et al. p53 expression as a prognostic marker in inflammatory breast cancer. Clin Cancer Res. 2004;10:6215-6221.
28. Cabioglu N, Gong Y, Islam R, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18:1021-1029.
29. van Golen KL, Wu ZF, Qiao XT, et al. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2:418-425.
30. Muss HB, Berry DL, Cirrincione C, et al. Standard chemotherapy (CMF or AC) versus capecitabine in early-stage breast cancer (BC) patients aged 65 or older: results of CALGB/CTSU 49907 [abstract]. J Clin Oncol (meeting abstracts). 2008;26(May 20 suppl):507.
31. Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381-5387.
32. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663-1671.
33. Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431-1439.
34. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable Her2-positive breast cancer. N Engl J Med. 2005;353:1673-1684.
35. Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16-21.
36. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386-3395.
37. Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101-2109.
38. O’Shaughnessy J, Blackwell KL, Burstein H, et al. A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progression on trastuzumab therapy [abstract]. J Clin Oncol (meeting abstracts). 2008;26(May 20 suppl):1015.
39. Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer: the M. D. Anderson Cancer Center experience. Clin Breast Cancer. 2004;4:415-419.
40. Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091-5097.
41. American Cancer Society. Survival rates for inflammatory breast cancer. www.cancer.org/cancer/breastcancer/moreinformation/inflammatorybreastcancer/inflammatory-breast-cancer-inflammatory-br-ca-aggressive. Accessed August 5, 2013.
To comment on this article, contact rdavidson@uspharmacist.com.





