US Pharm. 2013;38(10)(Diabetes suppl):11-17.
ABSTRACT: Patients at a high risk of developing type 2 diabetes mellitus are considered prediabetic. Unfortunately, the strict meaning of prediabetes has not been clearly defined, and cut-off values vary between organizations. This has made it difficult to obtain epidemiologic data as well as draw up diagnostic and screening schedules. Insulin resistance and beta-cell dysfunction remain central to the development of this condition. The treatment of prediabetes is vital in delaying the progression to full type 2 diabetes and includes lifestyle modifications as well as the use of antihyperglycemic drugs. This article discusses the different definitions of the term prediabetes, its pathophysiology, possible diagnostic measures, and current options available for treatment.
Prediabetes is a term used to describe a high-risk state for the development of diabetes.1 Individuals with prediabetes have higher plasma glucose than the normal range but lower than that in type 2 diabetes mellitus (T2DM).2 Higher plasma glucose can be described as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). Both IFG and IGT are risk factors for T2DM, and even more so when they occur together.2 Since not all individuals with prediabetes progress to T2DM, the use of this term in its strictest sense has been questioned, and some prefer to use the term intermediate hyperglycemia. According to the American Diabetes Association, prediabetic patients can be classified as shown in TABLE 1.3 Anything below these figures is classified as normal and above as diabetic.1,3

The World Health Organization (WHO) has a slightly different classification. According to its criteria, patients with one of the following states are described as prediabetic4:
- Those with an IFG of ≥6.1 and <7.0 mmol/L, without IGT, or
- Those with an IGT of <7.0 mmol/L and a 2-hour postload plasma glucose concentration of ≥7.8 and <11.1 mmol/L, measured during a 75-g oral glucose tolerance test (OGTT).
Epidemiology
It is difficult to obtain accurate epidemiologic data on the prevalence of the condition due to the varying definitions used to classify individuals with elevated glycemic risk.5 It has been observed that glucose measures that define IFG and IGT are able to identify about 10% of the adults who have prediabetes.6 Unfortunately, measures based on glycated hemoglobin (A1C) levels identify a significantly lower proportion of the population.6 There is a fair amount of evidence to demonstrate that glucose levels lower than those meeting the current definition of prediabetes may also be associated with similar risks, particularly in high-risk individuals.7
Epidemiologic data from the CDC estimate that 79 million adults aged 20 years and older in the U.S. had prediabetes in 2010, based upon fasting plasma glucose (FPG) and A1C levels.8 Its prevalence is on the rise, with an estimated 470 million people worldwide having prediabetes by 2030.1
It has been proposed that 5% to 10% of individuals with prediabetes will progress to diabetes annually, and about the same number will convert back to normoglycemia.1 Furthermore, patients with prediabetes have a 30% increased risk of developing T2DM over 4 years and a 70% risk over 30 years.9,10
Prediabetes is not only important in the effects it has on the development of T2DM but also as a risk factor of cardiovascular disease. It has been shown that prediabetes has a minor impact on microvascular disease and carries some predictive power for macrovascular disease.2 The increase in risk of cardiovascular disease seems to be multifactorial, involving a range of etiologies such as insulin resistance, hyperglycemia, dyslipidemia, hypertension, systemic inflammation, and oxidative stress.1 Insulin resistance also seems to be a common factor in obesity and prediabetes, two conditions that often coexist.2 Furthermore, prediabetes has been associated with early forms of nephropathy, chronic kidney disease, small fiber neuropathy, and diabetic retinopathy.1
It is interesting to note that contrary to previous information, the most recent data from a California study show that prediabetes is not an independent risk factor for cardiovascular events.11 This conflicting evidence remains to be clarified through further trials. For the purposes of this review, it is assumed that prediabetes is indeed a risk factor for cardiovascular disease.
Risk Factors
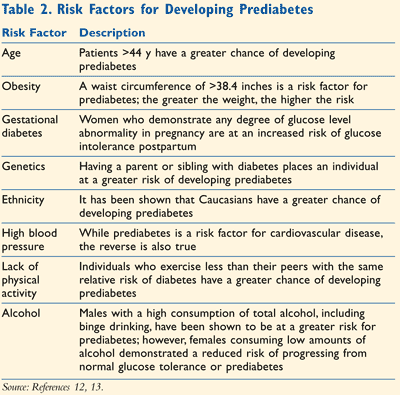
Various factors have been shown to increase a patient’s risk of developing prediabetes. These are very similar to the risk factors identified for diabetes and are listed in TABLE 2.12,13
Pathophysiology
The underlying pathophysiologic changes observed in prediabetes are very similar to those that lead to the development of T2DM.14 The characteristic insulin resistance as well as a genetic predisposition, increased insulin secretory demand, glucotoxicity, lipotoxicity, impaired incretin release/action, amylin accumulation, and decreased beta-cell mass play a causative role in the progressive beta-cell dysfunction characteristic of prediabetes.15
Insulin resistance is known to begin many years before the onset of diabetes, and decreased beta-cell function is already present in the prediabetic state.16,17 One study discovered that insulin sensitivity was reduced 13 years before the onset of diabetes, with the greatest reduction noted 5 years before the actual diagnosis of the condition. In this trial, it was shown that beta-cell function showed a compensatory increase 3 to 4 years before diagnosis and then decreased steeply.18
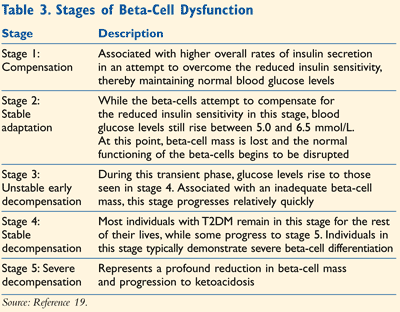
Based upon the changes in beta-cell mass, phenotype, and function observed as a patient moves toward the development of T2DM, a stepwise model has been proposed.19 It is postulated that the third stage in this model correlates to prediabetes in the early phase and to the manifestation of diabetes in the later phases. This model is summarized in TABLE 3.19
Free fatty acids (FFAs) play a role in prediabetes through various mechanisms. They stimulate the production of glucose in the liver, interfere with the extraction of insulin by the liver, and disrupt the insulin stimulated glucose uptake, phosphorylation, and oxidation in skeletal muscles. Increased lipolysis, which promotes the delivery of FFAs from the adipose tissue to skeletal muscle and the liver, is seen in obese patients, accentuating all of the previously mentioned effects.20
Diagnosis and Screening
Prediabetes is asymptomatic, and most patients are not even aware that they have the condition. Screening and diagnosis are vital in delaying or, better still, preventing the progression to T2DM. However, the fact that organizations cannot come to a consensus on the diagnostic criteria to define prediabetes complicates this process.21
Screening programs should be directed toward high-risk patients, including those with a body mass index (BMI) >35 kg/m2, systolic blood pressure ≥130 mmHg, or age older than 55 years. The glucose challenge test is recommended as the first line of screening since it is relatively cheaper than other tests currently available.22
One of the first screening tools developed was a simple tool that utilized only the information known to an average individual and avoided complex calculations.9 It was presented as a questionnaire including questions on the patient’s age, family history, waist circumference, any history of gestational diabetes, height, race, comorbid conditions, particularly hypertension, and level of physical activity. Using this information, health care professionals classify patients into those with undiagnosed diabetes, prediabetes, or neither undiagnosed diabetes nor prediabetes.
Diagnosis allows the identification of patients who may benefit the most from a diabetes prevention program.23 The IFG and IGT parameters relate to different segments of the at-risk population and different pathologic mechanisms.1,23 Furthermore, an FPG does not preclude an elevated OGTT and hence cannot rule out the presence of prediabetes.24
The Diabetes Prevention Program (DPP) study showed that 87% of new diabetes cases were diagnosed on the basis of an elevated 2-hour postchallenge plasma glucose compared to the 34% who were diagnosed based upon their initial elevated FPG levels.25 Therefore, an OGTT is essential in ensuring that all population groups are covered.23 A recent study conducted on African Americans demonstrated that FPG testing alone is not sufficient to diagnose prediabetes and recommends the use of OGTT until an alternative strategy is developed.26 The results of the various tests can be used to categorize patients into normal, prediabetic, or diabetic (TABLE 1).1,3
OGTT: This test measures the level of glucose in the blood 2 hours after a glucose load using 75-g anhydrous glucose dissolved in water.3
Fasting Plasma Glucose: FPG is the measure of blood glucose levels following an 8-hour fast. While this method it somewhat effective, it has been shown to miss the detection of approximately 30% of cases of T2DM, necessitating the use of the OGTT.27
A1C: The A1C test gives an average of the plasma glucose concentration over prolonged periods of time, usually around 3 to 4 months, with higher levels of A1C being observed in patients with elevated glucose levels. The test, which has to be performed in a laboratory, does not require a fasting state, has low intraindividual variability, and is a good predictor of diabetes-related complications. However, the limited data available on its usefulness in children and adolescents highlight its low sensitivity and specificity in this population, warranting the need for other testing methods.28 When being used as a target for therapeutic effectiveness, A1C levels should be reduced and maintained below 7%. Anything higher than this signals the need to initiate or alter therapy.24
Management
It is vital to effectively manage prediabetes in order to prevent and delay its progression to T2DM. The aim of therapy is to preserve insulin sensitivity and delay or prevent beta-cell failure.15,29
Lifestyle modifications including physical activity, dietary changes, and weight loss are recommended as first-line therapy.30 If the patient’s glucose levels cannot be brought down through these conservative measures, pharmacologic therapies are indicated. These comprise both diabetic and antidiabetic drugs, although the use of antihyperglycemic agents in this setting remains a matter for debate.30,31 It is useful to note that there are no FDA-approved drugs for prediabetes—all drugs are used off label.
Lifestyle Intervention: There is limited literature on the role of lifestyle interventions in prediabetes; however, it is safe to assume that data from a diabetic population are applicable to a prediabetic one. Therefore, although this section talks about the effects of lifestyle interventions in diabetes, the same rules apply in prediabetes. Obesity and physical inactivity have been identified as the two most important modifiable risk factors for diabetes, factors that can be changed through lifestyle intervention.10,32-34 Obesity has been recognized as the single most important factor in the epidemic increase in T2DM worldwide.
Weight loss, when achieved through increased physical activity, dietary modifications, or pharmacologic agents, is often difficult to maintain over the long term and is associated with weight regain in many cases.35 Furthermore, even in patients who achieve weight loss, only 50% to 60% demonstrate a decrease in the incidence of diabetes. This warrants the need for pharmacologic interventions in a number of patients.
Antidiabetic Drugs
As opposed to behavioral modification, pharmacologic therapy with antidiabetics achieves a uniform reduction in the IGT and IFG conversion to T2DM.35
Biguanides: Metformin reduces the FPG and A1C and has been proven to be safe by trial evidence, showing only mild gastrointestinal (GI) effects and no serious adverse effects.1,35 It exerts its effects by inhibiting hepatic glucose production and improving insulin sensitivity.1 However, metformin does not stimulate the secretion of insulin or preserve beta-cell function, and following an initial decline, A1C levels increase gradually.36-38 Metformin has been shown to demonstrate a greater beneficial effect in prediabetic patients with a higher baseline BMI and higher FPG than in their leaner counterparts with lower FPG concentrations.10
Thiazolidinediones (TZDs): These drugs (e.g., rosiglitazone, pioglitazone) exert their effect through the peroxisome proliferator activated receptor-γ (PPARγ).39 They are potent insulin sensitizers in muscle, liver, and adipocytes and exert a potent effect in enhancing and preserving beta-cell function.35 Additional studies have shown that TZDs cause a durable A1C reduction in T2DM and markedly reduce IGT conversion to T2DM.35
Alpha-Glucosidase Inhibitors: These agents (e.g., acarbose, miglitol) work by reducing the rate of polysaccharide digestion from the gut and have been shown to augment incretin secretion.40 Acarbose, in particular, reduces IGT conversion to T2DM.41 Furthermore, it has been proposed that alpha-glucosidase inhibitors exert a beneficial effect on glucose tolerance by modifying the gut microbiota flora.42 Although these agents do little to reduce A1C in patients with diabetes, they may have a greater role to play in prediabetes, as there is evidence to show that they decrease cardiovascular disease and the risk of hypertension in treated IGT patients.41 Acarbose may cause GI side effects, including flatulence and diarrhea.41
Glucagon-like Peptide-1 (GLP-1) Agonists: Exenatide and liraglutide are GLP-1 receptor agonists that mimic the actions of GLP-1 and are resistant to dipeptidyl peptidase-IV degradation. In the body, GLP-1, together with glucose-dependent insulinotropic polypeptide (GIP), accounts for 90% of the incretin effect. Both these drug entities produce sustained weight loss in obese patients and have been associated with an increased reversion from prediabetes to normoglycemia.1 The most frequent side effects observed are nausea and vomiting.
There are no established doses for the use of antidiabetic drugs in the treatment of prediabetes. TABLE 4 lists the currently employed doses of these drugs for the treatment of hypoglycemia in T2DM.39,43-49

Role of the Pharmacist
This article highlights the importance of the appropriate management of a prediabetic state to prevent or delay T2DM. Community pharmacists play a vital role in targeted screening by educating patients on the condition and encouraging high-risk individuals to undergo thorough diagnostic testing.50 The analysis of patient drug use by the pharmacist can provide leads to which patients should be counseled. The effectiveness of this strategy to identify high-risk patients has been substantiated through a recent trial.50 Additionally, support can be provided for patients undergoing lifestyle modifications as well as those on pharmacologic therapy. Community pharmacists can incorporate prediabetes awareness programs and interventions as an important element of medication therapy management.
REFERENCES
1. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279-2290.
2. Grundy S. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635-643.
3. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(suppl 1): S62-S69.
4. World Health Organization (WHO), International Diabetes Foundation (IDF). Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation.
Geneva, Switzerland: WHO; 2006.
www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf.
Accessed June 5, 2013.
5. James C, Bullard KM, Rolka DB, et al. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care. 2011;34:387-391.
6. Colagiuri S. Epidemiology of prediabetes. Med Clin North Am. 2011;95:299-307.
7. Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes Metab Res Rev. 2010;26:3-6.
8. The facts about diabetes: a leading cause of death in
the U.S. National Diabetes Education Program.
http://ndep.nih.gov/diabetes-facts/. Accessed June 5, 2013.
9. Heikes KE, Eddy DM, Arondekar B, Schlessinger L.
Diabetes Risk Calculator: a simple tool for detecting undiagnosed
diabetes and pre-diabetes. Diabetes Care. 2008;31:1040-1045.
10. Knowler WC, Barrett-Connor E, Fowler SE, et al;
Diabetes Prevention Program Research Group. Reduction in the incidence
of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
11. Deedwania P, Patel K, Fonarow GC, et al. Prediabetes
is not an independent risk factor for incident heart failure, other
cardiovascular events or mortality in older adults: findings from a
population-based cohort study. Int J Cardiol. June 3, 2013; Epub
ahead of print.
www.internationaljournalofcardiology.com/article/S0167-5273(13)00947-9/abstract.
Accessed June 10, 2013.
12. Retnakaran R, Qi Y, Sermer M, et al. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026-2031.
13. Cullmann M, Hilding A, Östenson CG. Alcohol
consumption and risk of pre-diabetes and type 2 diabetes development in a
Swedish population. Diabet Med. 2012;29:441-452.
14. Lee M, Saver JL, Hong KS, et al. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;7:344:e3564.
15. Bergman M. Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine. 2013;43:504-513.
16. Abdul-Ghani MA, Tripathy D, DeFronzo RA.
Contributions of beta-cell dysfunction and insulin resistance to the
pathogenesis of impaired glucose tolerance and impaired fasting glucose.
Diabetes Care. 2006;29:1130-1139.
17. Gastaldelli A, Ferrannini E, Miyazaki Y, et al; San
Antonio Metabolism study. Beta-cell dysfunction and glucose intolerance:
results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31-39.
18. Tabak AG, Jokela M, Akbaraly TN, et al. Trajectories
of glycaemia, insulin sensitivity, and insulin secretion before
diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215-2221.
19. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(suppl 3):S16-S21.
20. Diabetes Pro. American Diabetes Association.
http://professional.diabetes.org/Disease_Backgrounder.aspx?TYP=6&MID=256.
Accessed June 3, 2013.
21. Hollander P, Spellman C. Controversies in prediabetes: do we have a diagnosis? Postgrad Med. 2012;124:109-118.
22. Chatterjee R, Narayan KM, Lipscomb J, et al.
Screening for diabetes and prediabetes should be cost-saving in patients
at high risk. Diabetes Care. 2013;36:1981-1987.
23. Shaw J. Diagnosis of prediabetes. Med Clin North Am. 2011;95:341-352.
24. Fonseca VA. Identification and treatment of prediabetes to prevent progression to type 2 diabetes. Clin Cornerstone. 2007;8:10-8; discussion 19-20.
25. Diabetes Prevention Program Research Group 2007. The
prevalence of retinopathy in impaired glucose tolerance and recent-onset
diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137-144.
26. Cheng C, Kushner H, Falkner BE. The utility of fasting glucose for detection of prediabetes. Metabolism. 2006;55:434-438.
27. Aroda VR, Ratner R. Approach to the patient with prediabetes. J Clin Endocrinol Metab. 2008;93:3259-3265.
28. Nowicka P, Santoro N, Liu H, et al. Utility of
hemoglobin A1c for diagnosing prediabetes and diabetes in obese children
and adolescents. Diabetes Care. 2011;34:1306-1311.
29. Garber AJ. Obesity and type 2 diabetes: which patients are at risk? Diabetes Obes Metab. 2012;14:399-408.
30. Shane-McWhorter L. Prediabetes in a nursing facility patient with renal insufficiency. Consult Pharm. 2010;25(suppl B):11-18.
31. Hsueh WA, Orloski L, Wyne K. Prediabetes: the importance of early identification and intervention. Postgrad Med. 2010;122:129-143.
32. International Expert Committee. International Expert
Committee report on the role of the A1C assay in the diagnosis of
diabetes. Diabetes Care. 2009;32:1327-1334.
33. Tuomilehto J, Lindstrom J, Eriksson JG, et al;
Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes
mellitus by changes in lifestyle among subjects with impaired glucose
tolerance. N Engl J Med. 2001;344:1343-1350.
34. Ramachandran A, Snehalatha C, Mary S, et al. The
Indian Diabetes Prevention Programme shows that lifestyle modification
and metformin prevent type 2 diabetes in Asian Indian subjects with
impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289-297.
35. DeFronzo RA, Abdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96:2354-2366.
36. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
37. UK Prospective Diabetes Study Group. Intensive
blood-glucose control with sulphonylureas or insulin compared with
conventional treatment and risk of complications in patients with type 2
diabetes (UKPDS 33). Lancet. 1998;352:837-853.
38. UK Prospective Diabetes Study. Effect of intensive
blood glucose control with metformin on complications in overweight
patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
39. Sweetman SC, ed. Martindale: The Complete Drug Reference. 34th ed. London, UK: Pharmaceutical Press; 2005 [electronic version].
40. Moriya R, Shirakura T, Ito J, et al. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358-E1365.
41. Chiasson JL, Josse RG, Gomis R, et al; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077.
42. Slack E, Hapfelmeier S, Stecher B, et al. Innate and
adaptive immunity cooperate flexibly to maintain host-microbiota
mutualism. Science. 2009;325:617-620.
43. Glucophage (metformin) package insert. Princeton, NJ: Bristol-Myers Squibb Co; January 2009.
44. Avandia (rosiglitazone) package insert. Research Triangle Park, NC: GlaxoSmithKline; May 2011.
45. Actos (pioglitazone) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; August 2012.
46. Precose (acarbose) package insert. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; March 2011.
47. Glyset (miglitol) package insert. New York, NY: Pfizer Inc; August 2012.
48. Byetta (exenatide) package insert. Princeton, NJ: Bristol-Myers Squibb Co; February 2013.
49. Victoza (liraglutide) package insert. Plainsboro, NJ: Novo Nordisk Inc; April 2013.
50. Simoens S, Foulon E, Dethier M, el at. Promoting
targeted screening for type 2 diabetes mellitus: the contribution of
community pharmacists. Diabet Med. 2005;22:812-813.
To comment on this article, contact rdavidson@uspharmacist.com.





