US Pharm. 2013;38(11)(Oncology suppl):3-6.
ABSTRACT: Anemia is a common complication in patients with cancer. It can be caused by either the tumor itself or the chemotherapeutic regimen used. The severity and prevalence of the condition varies based on a number of factors. Treatment is aimed at increasing the oxygen-carrying capacity of the blood, reducing fatigue, and improving the patient’s overall quality of life. Erythropoiesis-stimulating agents, iron supplementation, and red blood cell transfusions have all been recommended in different settings. While these have proven beneficial in some patients, the optimal therapeutic modality is yet to be established.
Anemia is a major cause of morbidity in patients with cancer, linked with poor physical performance, prognosis, and therapeutic outcome.1 According to the World Health Organization (WHO), anemia is defined as a hemoglobin (Hb) level of <12 g/dL in nonpregnant women and <13 g/dL in men ≥15 years.2
Epidemiology
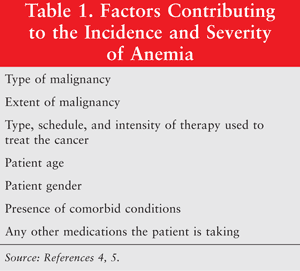
Anemia is one of the most frequent manifestations in patients with cancer. In fact, it has been shown that 30% to 90% of all cancer patients are anemic, a figure that is dependent upon the type of cancer and the definition of anemia used.3 The incidence and severity of the anemia depend upon a number of factors, which are listed in TABLE 1.4,5
Etiology
The main cause of anemia in cancer patients is twofold: the cancer itself and the therapy used for the management of the cancer. Cancer cells that infiltrate the bone marrow can directly suppress hematopoiesis and cause anemia. Furthermore, the cancer cells release cytokines that can lead to iron sequestration, reducing the production of red blood cells (RBCs). Tumors may result in chronic blood loss from the tumor site, leading to progressive anemia from the cancer and organ damage.6
This problem is compounded by blood losses, nutritional deficiencies, hemolysis, endocrine disorders, hereditary disease, renal insufficiency, or the presence of inflammatory cytokines associated with cancers and chronic disease.4,6 Cancer patients tend to lose their appetite, leading to nutritional deficiencies. In patients with gastrointestinal (GI) tumors, malabsorption of nutrients also leads to deficiencies. Hemolysis results from immune-mediated antibodies or changes in coagulation capability. As a result, anemia is a common comorbidity in patients recently diagnosed with cancer. Almost half of the patients diagnosed with gynecologic cancer, and one third of the patients diagnosed with non-Hodgkin lymphoma, have anemia at diagnosis.
Moreover, myelosuppression is one of the side effects of chemotherapy and a significant contributing factor to anemia. Radiation therapy to the skeleton in particular also causes hematologic toxicity and hence anemia.6
Symptoms
The most common symptoms of anemia are fatigue, dyspnea upon exertion, palpitations, depression, heart failure, impairment of cognitive function, dizziness, and heart failure.7,8
Diagnosis
The magnitude of the problem of anemia in cancer patients is amplified by suboptimal diagnostic measures, the lack of a standard definition, and the fact that the cause is often multi-factorial.6,9 A detailed history recording the duration and time to onset of the symptoms, presence of comorbid conditions, family history, and history of exposure to antineoplastic drugs and radiation should be taken. A physical examination to check for syncope, exercise dyspnea, headache, vertigo, chest pain, fatigue, abnormal menstruation in females, and pallor should be conducted.6
The National Cancer Comprehensive Network recommends that a CBC be performed for patients with an Hb <11 g/dL or a reduction in Hb count of 2 g/dL. This is useful in determining whether cytopenias are present. A visual review of the peripheral blood smear to confirm the size, shape, and color of the RBCs is also recommended.6
Pathophysiology
Anemia is the result of a combination of decreased circulating erythrocytes, reduced packed cell volume, and reduced Hb levels.6 This can occur through various pathophysiologic mechanisms including excess loss of blood, increased destruction of RBCs, and decreased erythropoiesis.
Erythropoietin (EPO), vitamin B12, folic acid, and iron are essential for erythropoiesis. Erythropoietin controls the maturation, differentiation, and survival of erythroid cells during this process.10 EPO is primarily produced by the kidneys and transported to the bone marrow.11
Abnormal iron metabolism or retention of iron in the macrophages reduces the amount of iron that is available for erythropoiesis. The altered metabolism of iron is primarily caused by hepcidin, a protein that decreases the absorption of iron in the intestines as well as the release of iron by the macrophages. The production of hepcidin is increased by cytokines released from tumors, thereby decreasing the amount of circulating iron. It has also been shown that the production of cytokines such as interleukin (IL)-6 is increased by certain chemotherapeutic regimens.5 Repeated cycles of chemotherapy may worsen the reduction in erythropoiesis cumulatively.7
As oxygen delivery is diminished, the body’s tissue cells become hypoxic, resulting in a series of physiological responses as the body attempts to gain or maintain tissue oxygenation.
It is thought that the normal utilization of iron in cancer patients may be interrupted by an interaction between the tumor cells and the host’s own immune system. This interaction leads to the up-regulation of specific inflammatory cytokines such as IL-1, gamma interferon, and tumor necrosis factor alpha (TNF-α), which decrease differentiation of erythroid precursors in the bone marrow, interfere with normal iron utilization, and inhibit normal hypoxia-driven erythropoietin production. Nephrotoxic chemotherapeutic agents may worsen the problem by causing renal impairment.
In chronic anemia, tumors have been shown to release an anemia-inducing factor, a molecule that reduces the life span of erythrocytes. This exacerbates anemia to the point where erythropoiesis cannot compensate for the inferior survival of the RBCs.12
Risk Factors for Anemia in Cancer
The following risk factors have been identified for anemia in cancer9:
- Myelosuppressive chemotherapy or radiation therapy
- Low Hb levels (10-12 g/dL) at the initiation of cytotoxic chemotherapy
- Administration of platinum-containing regimens, which also increases the need for transfusion support.
Treatment
Apart from an overall improvement in the patient’s quality of life, treatment of anemia is essential for a number of reasons. Anemia compromises the delivery of sufficient amounts of oxygen to all cells, including tumor cells.7 Since low tissue oxygenation is associated with a reduced sensitivity of tumors to radiation and other forms of chemotherapy, the efficacy of these treatments is reduced in anemia.13,14
The goal of therapy should be to increase the oxygen-carrying capacity of the blood and treat the underlying cause. Underlying conditions such as nutritional deficiencies are easier to treat than others, such as occult blood loss. Various options are available for the management of cancer patients with anemia. These include15:
- Appropriate supplemental therapy with folic acid and/or vitamin B12 to correct nutritional deficiencies
- Erythropoiesis-stimulating agents (ESAs)
- Iron supplementation
- Blood transfusions, which are indicated for patients with acute severe blood loss. For patients whose anemia is chronic, erythropoietic agents are preferred.
Erythropoiesis-Stimulating Agents: ESAs, including recombinant human erythropoietin alfa (rHuEPO; Procrit, Epogen) and darbepoetin alfa (Aranesp), were traditionally developed for the management of anemia in patients with chronic renal failure. They have demonstrated similar efficacy and limitations when used in comparable doses in cancer patients with anemia.5 ESAs are indicated for the management of patients whose endogenous EPO levels are abnormally low, as the ESA mode of action and immunologic and hematologic effects are similar to those of endogenous EPO.9 Studies conducted on rHuEPO have shown that it improves patients’ Hb levels and quality of life and decreases transfusion requirements.15,16 It has been proposed that ESAs also improve the cognitive function of patients receiving chemotherapy.17
A systemic review conducted by the American Cancer Society showed that optimal clinical benefit from erythropoietic treatment of chemotherapy-induced anemia may be achieved through early intervention.18 There is clearly a benefit of using ESAs; however, all other causes of anemia should be investi-gated and corrected prior to the use of ESA therapy.
Darbepoetin and rHuEPO are both indicated for the treatment of anemia in patients with nonmyeloid malignancies in whom anemia is related to chemotherapy.5 The American Society of Hematology (ASH) and the American Society of Clinical Oncology (ASCO) recommend that treatment be initiated when Hb levels drop below 10 g/dL and the dosage be reduced if there is a minimum increase of 1 g/dL in Hb levels by 2 weeks.19 The aim of the therapy should be to raise the Hb to the lowest concentration to avoid transfusion. If the minimal increase of 1 to 2 g/dL of Hb is not achieved after 6 to 8 weeks of treatment, the ESA therapy should be stopped.18 While it is difficult to predict patient response to these therapies with the current data, it has been shown that approximately 50% to 60% of patients demonstrate a 2 g/dL increase in Hb when treated with rHuEPO.9 ESA therapy should be discontinued upon completion of the chemotherapy course.20,21
The recommended dosage for darbepoetin alfa is 2.25 mcg/kg as a weekly subcutaneous (SC) injection. If the Hb levels do not rise by at least 1 g/dL after 6 weeks, the dosage should be increased to 4.5 mcg/kg. If the Hb levels increase more than 1 g/dL in a 2-week period or if Hb exceeds 12 g/dL, the dosage of darbepoetin should be reduced by 40%.20 Darbepoetin should be stopped if Hb levels rise above 13 g/dL.9
The recommended dosage of rHuEPO is 40,000 U administered SC once weekly or 150 U/kg administered SC three times a week in patients whose Hb levels are below 10 g/dL. The dosage of rHuEPO should be reduced by 25% if the Hb level increases >1 g/dL in any 2-week period or reaches a level needed to avoid RBC transfusion.21
ESAs are packaged in a vial that should not be shaken, as they will denature.20,21 The contents of the vial should not be diluted or pooled.
The risks of ESA therapy should also be taken into consideration. ESAs are thought to increase adverse cardiovascular events, enhance the risk of venous thromboembolism, shorten the time to tumor progression, and reduce survival in anemic cancer patients through different mechanisms.22,23 For this reason, the use of ESAs is discouraged in cancer patients who are not undergoing treatment with chemotherapy or radiotherapy.24 All ESAs come with a black box warning about increasing the risk of death, myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and tumor progression and recurrence.20,21 The ESA APPRISE Oncology Program has been set up by the FDA to advise patients, caregivers, and healthcare professionals on the safe use of ESAs.25
Iron Supplementation: Iron supplementation, preferably parenterally, is indicated in patients with low levels of ferritin (<30 ng/mL), transferrin saturation (TSAT <15%), and low (<26 pg/cell) reticulocyte Hb content (CHr). Two types of iron deficiency have been recognized in anemic cancer patients:
- Absolute iron deficiency (AID) that occurs with chronic bleeding due to, for example, GI or gynecologic lesions, blood loss from surgery, nutritional deficiencies, and anemia of chronic disease (ACD). It is defined as a serum ferritin level of <30 mcg/L and decreased TSAT of <15%.1
- Functional iron deficiency (FID) that arises after continued erythropoietin use. It is the most common cause of an inadequate response to ESA therapy, particularly in patients with renal failure. Therefore, most patients on ESA therapy will eventually require iron supplementation.6
Iron supplementation was introduced when ESA therapy alone demonstrated poor response rates in chemotherapy-treated patients.1 The efficacy of a combination of IV iron and ESA therapy was tested and the role of iron in functional iron deficiency was established.1 While current evidence supports the use of IV iron supplementation to improve hemoglobin responses in anemic patients with cancer, prospective studies on the long-term efficacy and safety of IV iron are needed. Furthermore, optimal dosing regimens need to be established.5
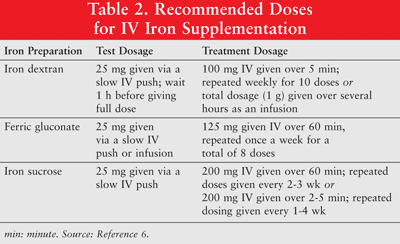
Iron can be administered orally or as parenteral formulations of low-molecular-weight (LMW) iron dextran, ferric gluconate, and iron sucrose. Parenterally administered iron has a superior response when compared to oral iron.6 The latter is therefore rarely used in cancer patients with anemia and is not discussed in this article. Test doses are recommended for iron dextran. Patients who have exhibited previous sensitivities to iron dextran or other IV iron preparations or those who have multiple drug allergies should also be administered a test dose. TABLE 2 shows the dosing regimen for IV iron supplementation.6
Parenteral iron is associated with hypotension, nausea, vomiting and/or diarrhea, pain, hypertension, dyspnea, pruritus, headache, and dizziness. Furthermore, it can be inconvenient to administer and costly.9 Since high-molecular-weight (HMW) iron dextran is associated with a higher incidence of adverse events than LMW iron dextran, the latter is preferred overall.6
Red Blood Cell Transfusion: RBCs are the preferred blood product for transfusions to correct anemia in cases of acute anemia after hemorrhage. Certain patients may require an infusion that is cytomegalovirus negative. The aim of using an RBC transfusion is to treat or prevent a deficiency in the oxygen-carrying capacity of the blood, and transfusion is not usually indicated if the patient’s Hb level is ≥10 g/dL.6
RBC transfusions offer the advantage of a rapid increase in Hb and hematocrit levels immediately upon infusion. It is estimated that a transfusion of 300 mL (1 unit) of pure RBCs will result in an average increase of 1 g/dL of Hb or 3% of hematocrit in an otherwise normal adult who does not experience simultaneous blood loss. This leads to an overall rapid improvement in fatigue.6
The patient’s blood must be cross-matched for ABO compatibility before the RBCs can be transfused. If the patient requires repeated infusions, the risk of adverse reactions can be minimized by the use of leukocyte-reducing blood and premedication with an antihistamine or acetaminophen.6
RBC transfusion is not without risks, including the potential for transfusion-transmitted infectious agents, transfusion reactions, alloimmunization, and immunosuppression.9 Other risks of transfusion include congestive heart failure, increased thrombotic events, and iron overload.6 It is useful to note, however, that patients requiring RBC infusions during the period corresponding to chemotherapy treatment only are highly unlikely to experience iron overload.6
Future Perspectives
Novel strategies for managing anemia in cancer patients are currently being pursued. While there is no substitute for human blood, researchers are in the process of developing products that have the oxygen-carrying capacity of RBCs. In the pipeline are Hb-based oxygen carriers and perfluorocarbons.5
Conclusion
All in all, the optimal approach to anemia as an important issue in oncology is yet to be established. Lack of consistency between healthcare providers, a clear definition of anemia in cancer, and diagnostic criteria all contribute to this problem.
REFERENCES
1. Steinmetz HT. The role of intravenous iron in the treatment of anemia in cancer patients. Ther Adv Hematol. 2012;3:177-191.
2. deBenoist B, McLean E, Egli I, et al. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anemia. Geneva, Switzerland: World Health Organization; 2008.
3. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(suppl 7A):11S-26S.
4. Schwartz RN. Anemia in patients with cancer: incidence,
causes, impact, management, and use of treatment guidelines and
protocols. Am J Health Syst Pharm. 2007;64(3 suppl 2):S5-S13; quiz S28-S30.
5. Calabrich A, Katz A. Management of anemia in cancer patients. Future Oncol. 2011;7:507-517.
6. Rodgers GM, Becker P, Blinder M, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2012;10:628-653.
7. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616-1634.
8. Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol. 2001;28:7-14.
9. Gillespie TW. Anemia in cancer. Cancer Nursing. 2003;26:119-128.
10. Koeller JM. Clinical guidelines for the treatment of cancer-related anemia. Pharmacotherapy. 1998;18:156-169.
11. Cazzola M, Mercuriali F, Brugnara C. Use of recombinant human erythropoietin outside the setting of uremia. Blood. 1997;89:424-867.
12. Nowrousian MR, Kasper C, Oberhoff C, et al.
Pathophysiology of cancer-related anemia. In: Smith JF, Boogaerts MA,
Ehmer B, eds. Erythropoietin in Cancer Supportive Treatment. New York, NY: Marcel Dekker; 1997:13-34.
13. Vaupel P, Dunst J, Engert A, et al. Effects of
recombinant human erythropoietin (rHuEPO) on tumor control in patients
with cancer-induced anemia. Onkologie. 2005;28:216-221.
14. Zhao KL, Liu G, Jiang GL, et al. Association of
haemoglobin level with morbidity and mortality of patients with locally
advanced oesophageal carcinoma undergoing radiotherapy—a secondary
analysis of three consecutive clinical phase III trials. Clin Oncol (R Coll Radiol). 2006;18:621-627.
15. Glaspy JA. The development of erythropoietic agents in oncology. Expert Opin Emerg Drugs. 2005;10:553-567.
16. Adamson JW, Spivak JL. Physiologic basis for the
pharmacologic use of recombinant human erythropoietin in surgery and
cancer treatment. Surgery. 1994;115:7-15.
17. Ferrario E, Ferrari L, Bidoli P, et al. Treatment of cancer-related anemia with epoetin alfa: a review. Cancer Treat Rev. 2004;30:563-575.
18. Lyman GH, Glaspy J. Are there clinical benefits with
early erythropoietic intervention for chemotherapy-induced anemia? A
systematic review. Cancer. 2006;106:223-233.
19. Rizzo JD, Brouwers M, Hurley P, et al. American
Society of Clinical Oncology/American Society of Hematology clinical
practice guideline update on the use of epoetin and darbepoetin in adult
patients with cancer. J Clin Oncol. 2010;28:4996-5010.
20. Aranesp (darbepoetin alfa) prescribing information. Thousand Oaks, CA: Amgen Inc; May 2012.
21. Procrit (epoetin alfa) prescribing information. Horsham, PA: Janssen Products, LP; July 2012.
22. Beutel G, Ganser A. Risks and benefits of erythropoiesis-stimulating agents in cancer management. Semin Hematol. 2007;44:157-165.
23. Fenner MH, Ganser A. Erythropoietin in cancer-related anemia. Curr Opin Oncol. 2008;20:685-689.
24. Smith RE Jr, Aapro MS, Ludwig H, et al. Darbepoetin
alfa for the treatment of anemia in patients with active cancer not
receiving chemo-therapy or radiotherapy: results of a phase III,
multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26:1040-1050.
25. ESA APPRISE Oncology Program. Amgen and Janssen Products, LP. www.esa-apprise.com. Accessed October 13, 2013.
To comment on this article, contact rdavidson@uspharmacist.com.





