HMG-CoA reductase inhibitors, more commonly known as statins, are some of the most widely prescribed medications in the United States.1 Their mechanism of action involves reducing low-density lipoprotein (LDL), or “bad” cholesterol. Statins are set apart from other LDL-lowering medications by the existence of multiple clinical trials showing a reduction in cardiovascular events that no other class of drugs targeting cholesterol has been able to replicate. It is proposed that statins have pleiotropic effects; that is, their actions in the body result in more than simply a reduction in LDL. Such actions include improvement of endothelial function, stabilization of atherosclerotic plaque, and inhibition of inflammation.2 Hence, because of these pleiotropic effects, statins may provide additional cardiovascular benefits; these too have not been replicated with other LDL-lowering drug classes. New guidelines for the treatment of hyperlipidemia have been published that may influence clinicians to utilize statins in more patients. This review summarizes the evidence, adverse-effect profiles, and cost of the different statins available as generic products in the U.S.
Major Clinical Trials of Generic Statins
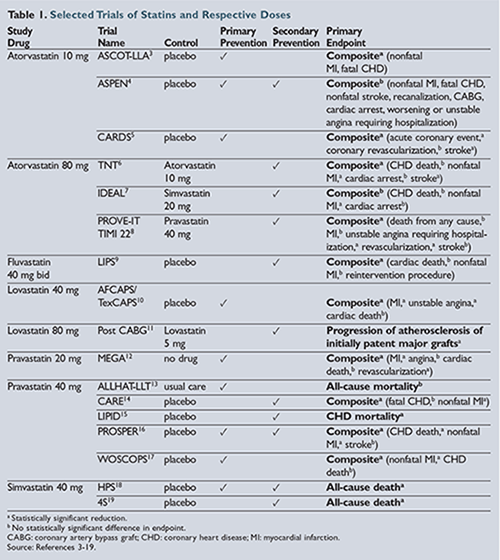
Several generically available statins were prospectively studied in the 1990s and 2000s to determine their cardiovascular benefits. The benefit of statin use in the prevention of cardiovascular events and death was replicated multiple times in clinical trials, some of which are highlighted in
Table 1
.3-19 Several trial methods were used, including placebo control groups, high-dose versus moderate-dose statins, usual care, and open-label studies. High-dose, high-intensity statin treatment was shown to have improved outcomes in patients who had experienced acute coronary syndromes or who had an established history of cardiovascular disease in the following trials: Treating to New Targets (TNT); High-dose Atorvastatin
vs Usual-dose Simvastatin for Secondary Prevention After Myocardial Infarction (IDEAL); and Pravastatin or Atorvastatin Evaluation and Infection Therapy—Thrombolysis in Myocardial Infarction (PROVE-IT TIMI 22)
.6-8 Primary prevention trials with moderate-intensity statins also highlighted the benefit of statin use in those patients at higher risk of developing cardiovascular disease. Several of these trials, including the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA); Collaborative Atorvastatin Diabetes Study (CARDS); Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints (ASPEN); and Heart Protection Study (HPS) included secondary analysis of diabetic cohorts.3-5
,18 While most of the original trials were not powered to identify statistical significance in these diabetic cohorts, there is a clear statistical trend showing that primary prevention of cardiovascular disease with statins in patients with diabetes improves outcomes as well.
The AHA and ACC Treatment and Management of Hyperlipidemia Guidelines
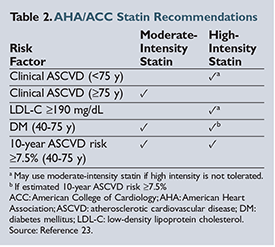
The American Heart Association (AHA) and the American College of Cardiology (ACC) published an update to the Adult Treatment Panel (ATP III) guidelines on the treatment and management of hyperlipidemia in November 2013.20 These guidelines focus on evidence-based approaches to managing hyperlipidemia and reducing the risk of atherosclerotic cardiovascular disease (ASCVD) almost exclusively through the use of statins. The authors stress the importance of focusing on intensity of statin therapy to achieve cardiovascular benefit rather than targeting specific LDL goals. The primary outcome of statin trials was the impact of a target statin dose and the subsequent reduction in cardiovascular death, nonfatal myocardial infarction (MI), and/or stroke. Critics of the new guidelines suggest that by following these recommendations, more people meet the criteria for receiving statin therapy through overestimation of the provided ASCVD risk-factor calculator.21 ,22
As shown in Table 2, virtually all patients with diabetes mellitus (DM), a baseline LDL above 190 mg/
dL, or a history of
ASCVD
should be treated with a statin. Using the ACC/AHA guideline risk-factor calculator, any white male over the age of 63 (over 66 years for African American men) or any white female over the age of 71 years (over 70 years for African American women) with optimal risk-factor levels should be taking a statin.23 If a person has hypertension, high LDL, low high-density lipoprotein (HDL), or is a current smoker, statins are recommended at younger
ages.
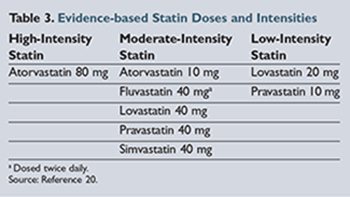
Along
with the recommendation to initiate a statin in these patients are further recommendations for the intensity of statin therapy based on observed LDL reduction (low, moderate, or high).
Table 3
lists the different statins and their respective intensity as defined by the new guidelines.20
Since adoption of the new guidelines may result in an increase in the number of prescriptions for statins, adverse effects and cost are likely to be of concern to many patients.
Adverse Effects of Statins
Despite the abundance of evidence to support the cardiovascular benefit of statins, a common concern of patients and providers is the potential for adverse events with statin use. Myalgia, myopathy, and the rare incidence of rhabdomyolysis are well-known adverse effects of statins.24 Surprisingly, adverse-event rates with statins were not statistically significant in most placebo-controlled studies.25 A meta-analysis was conducted in 2006 to identify potential adverse effects unnoticed by trial investigators.25 It was determined that statin use was associated with an increased risk of myopathy-related events, elevation in creatine phosphokinase, and elevation in liver function tests as compared to placebo. However, the authors note that for 1,000 patients treated with a statin, 37 cardiovascular events would be prevented and 5 adverse events would occur. It was also noted that in the included trials, the number needed to harm (NNH) for statin-related myopathy was 3,400 and the NNH for rhabdomyolysis was 7,428. The likelihood of any adverse events seemed to be highest with atorvastatin, followed by an equal risk with simvastatin, pravastatin, and lovastatin. Fluvastatin seemed to have the lowest risk.25
The risk of incident DM with statin therapy began to surface in the mid-2000s, particularly when the JUPITER trial investigators noted a statistically significant increase of newly diagnosed DM in the rosuvastatin arm.26 Several meta-analyses were conducted to identify the significance of the risk of DM in trials investigating all statins.27-30 A meta-analysis published in 2013 concluded a low risk of new-onset DM with pravastatin 40 mg, a moderate risk with atorvastatin 80 mg, and the highest risk with rosuvastatin 20 mg, though none of the observed risks were statistically significant.28 A second meta-analysis published in 2013 compared the relative risk of incident DM between pravastatin and all other statins.29 These authors found atorvastatin, rosuvastatin, and simvastatin to have a statistically significant relative risk increase in new-onset DM compared to pravastatin. While the results of the meta-analyses seemed to show that statin users have a significantly increased risk of DM, the benefits of preventing cardiovascular events and death outweigh this risk.27-30
Cost Comparisons of Generic Statins
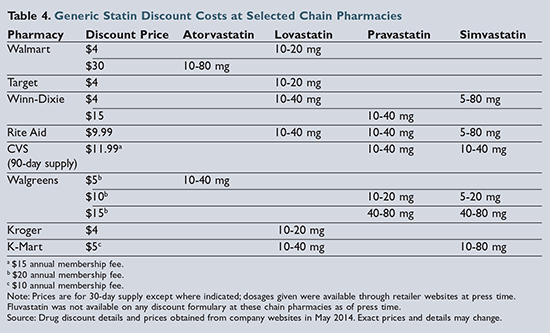
Of the six statins demonstrating clinical evidence for prevention of cardiovascular events and/or death, five are available generically. As of press time, lovastatin is the only option available on the lowest-cost generic formularies (i.e., the “4-dollar list”) from chains such as
Walmart and Target. Pravastatin was previously included on these formularies but was removed in January 2014. Pravastatin and simvastatin are included on some regional pharmacy-chain formularies, but pravastatin tends to be more expensive than either lovastatin or simvastatin, as shown in
Table 4
.
Though atorvastatin became available as a generic product in 2012, it is still relatively more expensive compared to the other generic statins.31 Walmart has begun advertising 30-day supplies of atorvastatin for $30; however, this is still significantly more expensive than alternative statins. Unfortunately, atorvastatin is the only generic statin included in the high-intensity treatment category. Therefore, a patient with coronary heart disease (CHD) or a history of a cardiovascular event who has financial limitations and requires treatment with a high-intensity statin may be limited to a moderate-intensity treatment option.
In March 2013, a settlement agreement was made between AstraZeneca, the manufacturer of Crestor ( rosuvastatin), and the pharmaceutical company Actavis for rights to manufacture generic rosuvastatin.32 As part of the settlement, Actavis is authorized to begin manufacturing rosuvastatin on May 2, 2016.
Conclusion
Statins have made a significant impact in the prevention of cardiovascular disease and cardiovascular death, as shown in several clinical trials. Generic availability of statins allows patients to afford treatment. However, cost may still be an issue if a high-intensity statin, such as atorvastatin, is indicated.
REFERENCES:
1. IMS Health.
National Prescription Audit. Dec 2012. Top 25 medicines dispensed by prescriptions (U.S.). Updated March 22, 2013.
www.imshealth.com. Accessed May 7, 2014.
2. Liao JK,
Laufs U. Pleiotropic effects of statins.
Annu Rev
Pharmacol
Toxicol. 2005
;45:89-118.
3. Sever PS,
Dahlöf B,
Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a
multicentre
randomised controlled trial. Lancet. 2003
;361:1149-1158.
4.
Knopp RH,
d’Emden M,
Smilde JG,
Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type
2 diabetes: the Atorvastatin Study for Prevention of coronary heart disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006
;29:1478-1485.
5.
Colhoun HM,
Betteridge DJ,
Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS):
multicentre
randomised placebo-controlled trial. Lancet. 2004
;364:685-696.
6. Shepherd J, Barter P,
Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006
;29:1220-1226.
7. Pedersen TR,
Faergeman O,
Kastelein JJ, et al. High-dose atorvastatin
vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005
;294:2437-2445.
8. Cannon CP,
Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N
Engl J Med. 2004
;350:1495-1504.
9.
Serruys PW, de
Feyter P,
Macaya C, et al.
Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002
;287:3215-3222.
10. Downs J, Clearfield M, Weis S,
et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels.
Results of AFCAPS/
TexCAPS.
Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998
;279:1615-1622.
11.
Hoogwerf BJ,
Waness A,
Cressman M, et al. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: the Post Coronary Artery Bypass Graft Trial. Diabetes. 1999
;48:1289-1294.
12. Nakamura H, Arakawa K,
Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective
randomised controlled trial. Lancet. 2006
;368:1155-1163.
13. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately
hypercholesterolemic, hypertensive patients randomized to pravastatin
vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002
;288:2998-3007.
14. Sacks FM,
Pfeffer MA,
Moye LA, et al.
The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels.
Cholesterol and Recurrent Events Trial investigators. N
Engl J Med. 1996
;335:1001-1009.
15. The Long-term Intervention with Pravastatin in
Ischaemic Disease (LIPID) Study Group.
Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N
Engl J Med. 1998
;339:1349-1357.
16. Shepherd J,
Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a
randomised controlled trial. Lancet. 2002
;360:1623-1630.
17. Shepherd J,
Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia.
West of Scotland Coronary Prevention Study Group. N
Engl J Med. 1995
;333:1301-1307.
18. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a
randomised placebo-controlled trial. Lancet. 2002
;360:7-22.
19.
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994
;344:1383-1389.
20. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am
Coll
Cardiol. Published November 12, 2013 [
Epub ahead of print]
;
pii: S0735-1097(13)06028-2. https://content.onlinejacc.org/article.aspx?articleid=1770217. Accessed May 7, 2014.
21.
Ades PA. A controversial step forward: a commentary on the 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults.
Coron Artery Dis. 2014; 25:360-363.
22. Seth B, Williams JS. Recent AHA/ACC cholesterol guidelines: vice or virtue? Metabolism.
2014; 63:605-606.
23. American College of Cardiology/American Heart Association.
2013 Prevention Guidelines Tools—CV Risk Calculator. http://my.americanheart.org/cvriskcalculator. Accessed May 7, 2014.
24.
Sathasivam S, Lecky B. Statin induced myopathy. BMJ. 2008
;337:a2286.
25. Silva MA, Swanson AC, Gandhi PJ,
Tataronis GR. Statin-related adverse events: a meta-analysis.
Clin
Ther. 2006
;28:26-35.
26.
Ridker PM, Danielson E, Fonseca FA, et al.
Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N
Engl J Med. 2008
;359:2195-2207.
27.
Rajpathak SN,
Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009
;32:1924-1929.
28.
Navarese EP, Buffon A,
Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J
Cardiol
. 2013
;111:1123-1130.
29. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013
;346:f2610.
30.
Sattar N,
Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of
randomised statin trials. Lancet. 2010
;375:735-742.
31.
Truven Health Analytics Micromedex Clinical Knowledge Suite.
Red Book 2014. http://micromedex.com/redbook. Accessed May 6, 2014.
32.
Actavis reaches agreement with AstraZeneca to launch a generic version of Crestor in 2016 [press release]. Published March 25, 2013. http://ir.actavis.com/phoenix.zhtml
?c=65778&p=irol-newsArticle_Print&ID=1799778&highlight.
Accessed May 7, 2014.
To comment on this article, contact rdavidson@uspharmacist.com.