US Pharm. 2014;39(9)((Specialty&Oncology):8-11.
ABSTRACT: Late discovery, metastasis, stroma, multiple genetic mutations, and cancer stem cells are factors related to the poor prognosis of pancreatic cancer. Histologic examination of biopsied tissue, elevated serum lipase and amylase, cancer markers, and procedures such as magnetic resonance cholangiopancreatography confirm the diagnosis. Resection (Whipple procedure) and chemotherapy, sometimes combined with radiation therapy, are used to achieve the goals of remission and enhancement of overall survival. Monotherapy using 5-fluorouracil or gemcitabine, gemcitabine in combination with other therapies, combination therapy using FOLFIRINOX, and novel drug-delivery systems such as nab-paclitaxel have become cornerstones of treatment. Despite these methods, however, overall survival rates remain low.
Pancreatic cancer can develop in both the exocrine and the endocrine segments of the pancreas; however, the majority of cases are exocrine in origin. Pancreatic ductal adenocarcinoma (PDAC) is an aggressive, multifactorial, often metastatic cancer of the exocrine pancreas that generally originates in the epithelial cells of the large pancreatic ducts. It is commonly diagnosed when it has progressed extensively, resulting in poor patient prognosis.1 In comparison with other solid cancers of similar etiology (e.g., those of the lung, breast, and prostate), although pancreatic cancer has a relatively lower incidence (2.8% of all new cancer cases), overall mortality is much higher, with a 5-year survival rate of only 6.7%.2 In an attempt to reduce the mortality rate and increase overall survival, especially in metastatic pancreatic cancer patients with good performance status, a number of clinical trials involving differential monotherapy or combination therapies have been conducted, as discussed below. Unfortunately, remission and long-term improvements in overall survival remain largely elusive.
Etiology and Pathogenesis of Pancreatic Cancer
A plethora of exogenous and endogenous factors trigger the development of pancreatic neoplasia. Prime among exogenous triggers are chronic lifestyle behaviors such as cigarette smoking, alcoholism, and excessive consumption of lipid-rich foods.3 Persistent or recurrent exposure to these exogenous factors activates a sequential pattern of genetic mutations resulting in dysplastic metamorphosis of the pancreatic ductal epithelial cells and subsequent development of pancreatic adenocarcinoma.
Symptomatology and Diagnosis
The typical PDAC patient presents with symptoms such as abdominal pain, jaundice caused by obstruction of the common bile duct, and unexplained nausea and vomiting. Symptoms are frequently ignored by the patient for some time, as they are relatively nonspecific.4 Other possible symptoms include unexplained weight loss, fatigue, and depression.5,6
From a diagnostic standpoint, elevated serum amylase and lipase levels indicate a compromised pancreas. In addition, the tumor markers cancer antigen (CA) 19-9 and carcinoembryonic antigen (CEA) are useful for diagnosis, since many patients with PDAC have elevated levels. Although CT and MRI scanning are commonly used, the gold standard is histologic examination of a pancreatic biopsy.4 Only 20% of patients present with early-stage, localized disease. Approximately 40% present with locally advanced disease, and an equal percentage present with metastatic disease most commonly involving the regional lymph nodes, liver, and (less commonly) lungs.7
Clinical Management
As with all malignancies, the treatment approach is selected based upon the stage of disease and the patient’s status. Therapy typically involves interventional and pharmacologic modalities including resection and chemotherapy. In a resection, the damaged part of the organ is removed, and the alimentary canal is surgically reconstructed. Informally known as the Whipple procedure, this is an intensive operation requiring a specialized oncologist.
Radiation therapy in combination with chemotherapy may be beneficial at an early tumor stage, but even dose escalation of radiation may not result in augmentation of the overall survival rate, especially in patients with advanced unresectable PDAC.8 Xu et al performed a meta-analysis of 17 published studies involving a total of 3,088 patients to compare the effect of chemoradiotherapy and neoadjuvant chemoradiotherapy on overall survival rate in resectable pancreatic cancer. Neither therapy yielded improvements in overall survival, however.9 This underscores the difficulties of designing an optimal treatment program.
Chemotherapy: The Role of Gemcitabine
Until 1996, the nucleoside analogue 5-fluorouracil (5-FU) was extensively used for the treatment of PDAC. In 1997, Burris et al compared medical outcomes in patients treated with 5-FU and patients treated with the pyrimidine analogue gemcitabine. Although median patient survival following treatment was extended by only 1.24 months in the gemcitabine cohort versus the 5-FU group, 23.8% of gemcitabine patients showed a significant clinical response compared with 4.8% of 5-FU patients. Gemcitabine monotherapy significantly augmented 1-year survival by 18%, compared with 2% survival for 5-FU therapy.10 For about a decade following this trial, gemcitabine monotherapy remained the gold standard of treatment. It is still used extensively when the patient’s performance status is poor and more intensive therapy is unlikely to be tolerated.
Combination Therapies With Gemcitabine
The years between 1997 and 2006 saw PDAC therapy centered on enhancing gemcitabine cytotoxicity by synergism with other antineoplastic agents. TABLE 1 summarizes the combination-based clinical trials conducted from 2002 onward.11-21 While there have been some advances in attaining a progression-free state, therapy has not been effective overall. Despite marginal improvements in outcomes, many of these combination therapies are still commonly used, especially when other interventions fail.
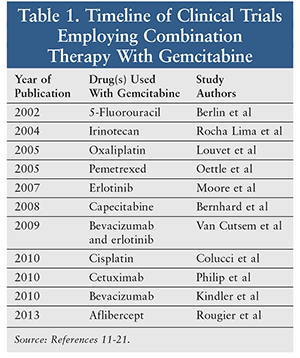
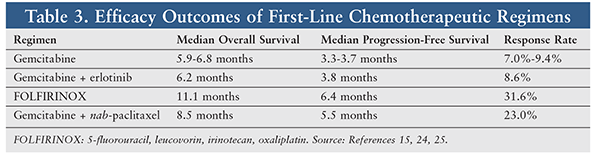
A study published by Moore et al in 2007 reported one of the most promising combinations. In this study, 569 patients with Eastern Cooperative Oncology Group (ECOG) performance status scores of 0 to 2 were randomized to gemcitabine alone or gemcitabine plus erlotinib. (The ECOG scale range is 0 to 5, with higher numbers indicating a greater burden of illness impacting ability to perform activities of daily living.) Median overall survival was slightly improved in the combination therapy arm (6.2 vs. 5.9 months), as were median progression-free survival (3.75 vs. 3.55 months) and response rate (8.6% vs. 8.0%).15
Interestingly, the use of combination therapy has prompted investigation into the use of natural compounds abundant in the diet as synergistic agents for exacerbating gemcitabine cytotoxicity in pancreatic cancer. However, most investigations have remained at the laboratory or animal-model stage of research.
Novel Drug Delivery Using Transarterial Infusion
A recent variation in the approach to gemcitabine combination therapy has concentrated on novel methods of drug delivery. The novelty lies in either the choice of invasive procedure for drug delivery or the invention of newer targeted-delivery pharmaceutical formulations. Chen et al performed transarterial infusions of gemcitabine and oxaliplatin in 32 patients with unresectable pancreatic cancer; therapeutic courses were repeated every 4 weeks, for a total of 105 cycles. This treatment resulted in a 25% overall response rate.22
FOLFIRINOX Regimen
In a 2005 noncontrolled study by Conroy et al, 46 therapy-naïve patients with locally advanced or metastatic PDAC were treated with the chemotherapeutic regimen FOLFIRINOX (5-FU, leucovorin, irinotecan, and oxaliplatin). The results were promising, with an overall response rate of 26%, median time to progression of 8.2 months, and median overall survival of 10.2 months.23 This prompted a larger controlled trial that randomized 342 patients to FOLFIRINOX or gemcitabine.24 Median overall survival in this trial was extended in the FOLFIRINOX group versus the gemcitabine group (11.1 vs. 6.8 months), as was median progression-free survival (6.4 vs. 3.3 months), and response rates were also improved (31.6% vs. 9.4%). However, chemotherapeutic toxicities were greater in the FOLFIRINOX group. It is important to note that only patients with ECOG performance status scores of 0 or 1 were included.24 This hallmark trial has led to widespread use of FOLFIRINOX in patients with good performance-status scores. However, FOLFIRINOX may not be an option for patients with greater severity of illness, because of higher rates of serious adverse reactions with this combination therapy.
The Novel Formulation nab-Paclitaxel
A newer drug delivery method, nab-paclitaxel (Abraxane), has emerged for the treatment of metastatic pancreatic cancer and is FDA-approved for this indication. Paclitaxel is an antimitotic compound that targets the cytoskeletal machinery required for cell reproduction. Recently, nanotechnology was used to devise a formulation wherein paclitaxel is bound to albumin protein nanoparticles (nanoparticle albumin-bound paclitaxel, or nab-paclitaxel). This formulation was found to simultaneously reduce toxicity and enhance drug delivery to the tumor site.
In 2013, the Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT), in which nab-paclitaxel was added to gemcitabine therapy, demonstrated improvements in overall survival. MPACT randomized 861 previously untreated metastatic pancreatic cancer patients to nab-paclitaxel followed by gemcitabine on days 1, 8, and 15 every 4 weeks or gemcitabine weekly for 7 weeks and then on days 1, 8, and 15 every 4 weeks. Median overall survival was improved in the combination group versus the monotherapy group (8.5 months vs. 6.7 months), as were median progression-free survival (5.5 months vs. 3.7 months) and overall response rate (23% vs. 7%).25 While greater toxicity was recorded in the gemcitabine plus nab-paclitaxel group, toxicity was less in comparison with the FOLFIRINOX regimen.
Current First-Line Treatment Strategies
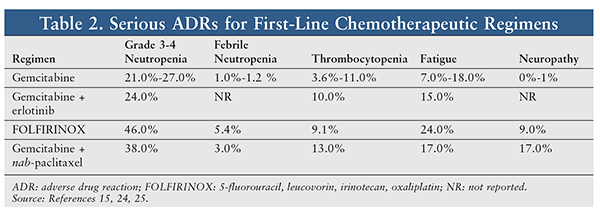
The National Comprehensive Cancer Network Guidelines recommend that selection of treatment be stratified by performance status. Patients with poor performance status (typically defined as ECOG performance-status scores 3) are limited to either gemcitabine monotherapy or palliative care. Patients with good performance status (ECOG performance-status scores 0-2) may elect treatment with FOLFIRINOX, gemcitabine plus either erlotinib or nab-paclitaxel, or gemcitabine monotherapy, or enrollment in a clinical trial.26 In selecting a regimen, potential toxicities and anticipated outcomes must be considered (TABLES 2 and 3).
Management of Chemotherapy-Induced Toxicity
Supportive care is of paramount importance in enabling patients to progress successfully through their treatment regimens. FOLFIRINOX is classified as having moderate emetogenic potential, but all other first-line regimens are considered to have low emetogenic potential. Antiemetic prophylaxis should be administered in accordance with recommendations from national guidelines. Patients receiving first-line regimens do not qualify for prophylactic use of colony-stimulating factors during their first cycle of chemotherapy, but may qualify during subsequent courses depending upon the clinical scenario.
Erlotinib-induced rash is a unique adverse reaction affecting 60% to 80% of recipients, with onset typically 10 to 14 days after initiation. Both preemptive and reactive management strategies for erlotinib-induced rash are acceptable. Preemptive therapy focuses on appropriate skin care with the addition of corticosteroid cream and oral doxycycline or minocycline. Reactive treatment includes skin care in combination with topical clindamycin gel or corticosteroid cream. For severe reactions, dose reduction or withholding of erlotinib, with addition of an oral steroid, is suggested.7 For management and prevention of other adverse events, refer to the National Comprehensive Cancer Network Guidelines (available at www.nccn.org).
Conclusion
Combination therapy, with the goal of optimizing cancer cell cytotoxicity while minimizing systemic toxicities, seems to have emerged as the primary clinical management strategy for responsive pancreatic adenocarcinoma. Drug regimens have concentrated on agents such as 5-FU and gemcitabine, but have expanded to include FOLFIRINOX and other gemcitabine-based regimens. Since prognosis and overall survival remain low, progressive therapeutic modalities will probably be a mix of technologically enhanced drug-delivery systems such as nab-paclitaxel; modern interventional, pharmacognostic, or pharmacologic products; and improvised radiation therapy and surgery. Early detection, coupled with individualized patient-centered treatment, may pave the way for therapeutic breakthroughs.
References
1. Ghosn M, Kourie HR, El Karak F, et al. Optimum chemotherapy in the management of metastatic pancreatic cancer. World J Gastroenterol. 2014;20:2352-2357.
2. National Cancer Institute. SEER stat fact sheets: pancreas cancer. http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed December 18, 2013.
3. Zheng W, McLaughlin JK, Gridley G, et al. A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States). Cancer Causes Control. 1993;4:477-482.
4. Hara Y, Abe M. Current status and problems in the diagnosis of pancreatic cancer. Kango Gijutsu. 1985;31:853-857.
5. Fazal S, Saif MW. Supportive and palliative care of pancreatic cancer. JOP. 2007;8:240-253.
6. Lee JH, Kim SA, Park HY, et al. New-onset diabetes patients need pancreatic cancer screening? J Clin Gastroenterol. 2012;46:e58-e61.
7. Campen CJ, Dragovich T, Baker AF. Management strategies in pancreatic cancer. Am J Health Syst Pharm. 2011;68:573-584.
8. Hall WA, Colbert LE, Nickleach D, et al. The influence of radiation therapy dose escalation on overall survival in unresectable pancreatic adenocarcinoma. J Gastrointest Oncol. 2014;5:77-85.
9. Xu CP, Xue XJ, Liang N, et al. Effect of chemoradiotherapy and neoadjuvant chemoradiotherapy in resectable pancreatic cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:549-559.
10. Burris HA III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413.
11. Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275.
12. Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776-3783.
13. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516.
14. Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639-1645.
15. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
16. Bernhard J, Dietrich D, Scheithauer W, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial—SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol. 2008;26:3695-3701.
17. Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237.
18. Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645-1651.
19. Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610.
20. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622.
21. Rougier P, Riess H, Manges R, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49:2633-2642.
22. Chen Y, Wang XL, Wang JH, et al. Transarterial infusion with gemcitabine and oxaliplatin for the treatment of unresectable pancreatic cancer. Anticancer Drugs. May 5, 2014 [Epub ahead of print].
23. Conroy T, Paillot B, François E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer—a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228-1236.
24. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825.
25. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703.
26. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Pancreatic adenocarcinoma (version 2.2014). www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed July 27, 2014.
To comment on this article, contact rdavidson@uspharmacist.com.





