US Pharm. 2014;39(11)(Specialty&Oncology suppl):3-7.
ABSTRACT: Rheumatoid arthritis requires early intervention to prevent long-term complications and morbidity. The exact trigger is unknown, but symmetrical synovitis in the small joints of the hands and feet is the primary presentation. Recently published guidelines featuring new, more aggressive treatment recommendations discuss disease-modifying antirheumatic drugs, new biologic agents, and methods of switching between them. The most effective medications have numerous side effects, particularly immunosuppression. In order to achieve higher response rates, it will be necessary to develop additional therapeutic options with novel mechanisms.
The treatment of RA has advanced over time, with the goals of early therapy and preservation of functional ability. In recent years, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) have released new and more aggressive recommendations for treatment. These recommendations address the role of disease-modifying antirheumatic drugs, incorporate additional biologic agents, and explain how to switch between these agents. This article will review the updated ACR guidelines, outline the therapeutic role of approved therapies, discuss appropriate vaccination recommendations, and highlight the current pharmaceutical landscape of pipeline agents. The impact of pharmacists on patient and disease-state management will also be discussed.
Disease Overview
Arthritic diseases affect a large percentage of the aging population, with osteoarthritis the most common form in adults; however, rheumatoid arthritis (RA) can be more debilitating and may afflict younger patients. Osteoarthritis most commonly affects weight-bearing joints such as the knees and hips and is not associated with other systemic complications; RA most commonly affects numerous joints in the hands and feet (although other joints can be affected) and leads to systemic complications in other organs.1
RA, a chronic autoimmune disease that affects approximately 0.5% to 1% of the general population, manifests as painful swelling and tenderness of the joints that can impair daily activities and limit productivity.1 RA not only contributes to significant disability and adversely affects quality of life, but also carries a significant socioeconomic burden.2 In 2003, the total cost attributable to arthritis and other rheumatic conditions in the United States was approximately $128 billion, with $47 billion in indirect costs (i.e., therapy costs, medical visits, complications of therapy, disease progression, lost earnings).3 These nonmedical and direct and indirect medical expenditures, along with the increasing use of new and expensive biologic therapies, highlight the need for healthcare systems to focus on cost-effective disease management. Therefore, it is important to evaluate the role of RA therapies following recent treatment updates, with emphasis on cost containment and the contribution of pharmacists to optimal medication use and positive patient outcomes.
Pathogenesis and Physical Presentation
The exact trigger of RA is unknown; however, the primary presentation is symmetrical synovitis in the small joints of the hands and feet. Patients experience pain, heat, stiffness, and reduced joint functionality caused by swelling of the synovial joint membranes.4 Progressive joint damage leads to bone deformity, classically known as pannus formation.4,5
Pathogenesis involves an autoimmune process in which cytokines, rheumatoid factor (RF), and anticitrullinated protein (anti-CCP) antibody production are stimulated by a CD4+ T-cell response.5,6 Increased blood flow to the synovial joints results in heat production and a surge in blood vessel formation. The overproduction and augmented size of synoviocytes lead to swelling as synovial fluid and membrane materials are produced.4 Owing to the massive inflammatory process, patients may exhibit fever, perspiration, and fatigue, and laboratory findings may include elevated erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP). In severe cases, vasculitis, peripheral neuropathy, or inflammation of the pleural or pericardial linings may develop. Many questions remain regarding the pathogenesis of RA, including the triggers (infectious and neuroimmune factors) and genetic factors contributing to the disease.5,6
RA can be characterized according to duration of disease and level of disease activity. Early RA and established RA are defined as disease duration of <6 months and ≥6 months, respectively.7 Disease activity is classified as low, moderate, or high according to factors including pain, number of affected joints, function, global patient status, and ESR.8 The 2012 ACR guideline provides revised treatment approaches based upon disease duration and activity. These updates include more aggressive therapy for early RA, with key components to improving outcomes being prompt recognition of synovitis and initiation of appropriate treatment to address inflammation.4,7 The 2013 EULAR recommendations divide treatment into three phases, outline steps for therapy escalation, and emphasize assessment of structural damage, comorbidities, and side effects, in addition to disease activity when modifying therapy.9
Additionally, the ACR guidelines make recommendations based upon the patient’s prognostic factors.7 The presence of poor prognostic factors—including bony erosions and extra-articular disease, poor physical-function score, and elevations in RF or anti-CCP—is associated with worse outcomes and early disability. Other factors, including older age, female sex, smoking, and elevated CRP and ESR, also contribute to poor prognosis.10
Therapeutics
Although treatment for early versus established RA differs according to disease severity, both guidelines set goals of therapy to achieve low disease activity or remission along with maintaining functionality and pain control.8 Conventional disease-modifying antirheumatic drugs (DMARDs) are preferred and should be initiated immediately after diagnosis, within 3 months of onset of persistent symptoms.7,9 This drug class includes methotrexate, leflunomide, hydroxychloroquine, minocycline, and sulfasalazine.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids are effective for symptomatic and functional improvement, especially during flares, and are used as bridge therapy to DMARDs. Monotherapy should be avoided and its use limited to the shortest duration and the lowest dosage that maintain remission. The adverse event (AE) profiles of both of these medication classes require monitoring for a wide array of complications.9-11
Biologicals used for RA are classified as antitumor necrosis factor (anti-TNF) agents and non-TNF agents. First-line medications are the anti-TNF agents, which include etanercept, infliximab, adalimumab, golimumab, certolizumab, and the Janus kinase (JAK) inhibitor tofacitinib.11 Second-line options include T-lymphocyte inhibitor, abatacept, interleukin (IL)-6 receptor inhibitor, tocilizumab, and the monoclonal antibody rituximab. The third-line choice is the IL-1 receptor antagonist anakinra.7 Administered alone, these agents exhibit efficacy comparable to that for methotrexate; however, their use in combination with methotrexate results in greater efficacy. Head-to-head studies have not been performed with different drugs in this class. The choice of drug is often determined by cost, dosing frequency, patient preference, and insurance formulary. If a patient does not respond to one agent, evidence supports switching to another biological.11
Because of their immunosuppressive effects, biologicals increase the risk of serious bacterial, viral, and fungal infections, especially in elderly patients. Tocilizumab, tofacitinib, and all anti-TNF agents carry a black box warning regarding increased risk of serious infections. All patients should undergo a purified protein derivative tuberculin test and be screened for hepatitis B status prior to starting a biological, and they should be monitored every few months to assess CBC and liver function. Patients taking biologicals are at increased risk for lymphomas and malignancies, central nervous system demyelination, heart failure, formation of autoantibodies to DNA, and development of antibodies against the agent itself. Common AEs include local site reactions, transaminitis, and hematologic effects such as thrombocytopenia and neutropenia. In general, a biological may be used in combination with nonbiologic DMARDs, glucocorticoids, and NSAIDs, but should not be combined with another biologic agent owing to the increased risk of serious AEs.4,7,9
Monitoring for response to therapy is accomplished through rating scales, disease activity scores (DAS), clinical status, and laboratory follow-up. Vectra DA is a newly approved test for monitoring the follow-up of RA patients that correlates multiple laboratory parameters to give an aggregate DAS. Patients should be monitored every 1 to 3 months, and treatment should be adjusted if there is no improvement after 3 months or if the treatment target has not been achieved in 6 months. Patients should also be assessed annually for disease activity and damage, functional ability, development of disease complications, and need for referral or surgery.9
The updated guidelines include three new agents, which are summarized in FIGURE 1.12,13
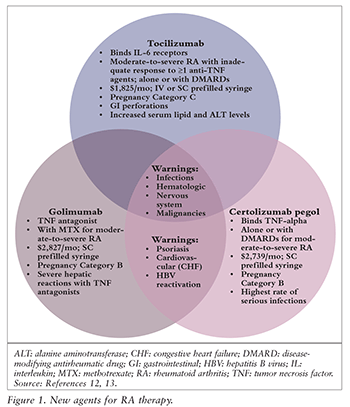
Initial Therapy
In early RA, patients should be started on DMARD monotherapy or combination therapy (TABLE 1). Methotrexate is the gold standard; however, if it is contraindicated or not tolerated, sulfasalazine, hydroxycholorquine, minocycline, and leflunomide may be used first-line.9 As adjunctive therapy, patients may be placed on short-term use of NSAIDs and low-dose glucocorticoids to provide symptomatic functional relief, although this relief diminishes after 1 year. Tapering of corticosteroids should be attempted in patients with stable disease.4,9 Although there is no difference in tolerability, DMARD combination therapy appears to be superior to monotherapy in terms of symptom management, quality of life, and ability to achieve remission and decelerate joint damage. Regardless of the DMARD strategy employed, the speed and intensity (escalation to therapeutic dose) of treatment is more important than the combination of drugs used.4 In patients with high disease activity and features of poor prognosis, an anti-TNF agent may be used alone or in combination with methotrexate, although the combination is superior to either agent alone.4,7
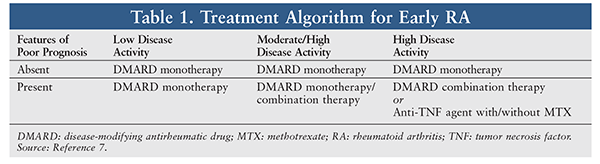
Established RA
Patients with established RA should be reassessed 3 months after any changes to therapy, or in 6 months if a non-TNF agent is being used. If disease activity does not improve, additional DMARDs should be added (e.g., monotherapy to double therapy to triple therapy), or a switch should be made to a different DMARD. For poor responders, an anti-TNF biological may be added also. Alternatively, for severely affected patients, the ACR recommends adding or switching to a biological such as an anti-TNF agent (abatacept, tocilizumab, or rituximab).7
If moderate or high disease activity persists owing to a lack or loss of benefit or a nonserious AE after 3 months of anti-TNF therapy or 6 months of non-TNF therapy, it is recommended to switch to another anti-TNF or non-TNF agent.7 If high disease activity is seen following failure of anti-TNF therapy due to a serious AE, the patient should be switched to a non-TNF agent.7 See FIGURES 2 and 3).
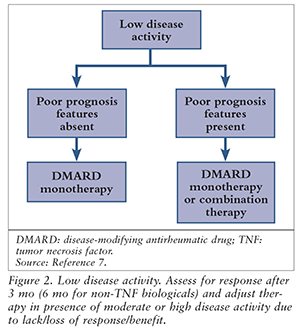
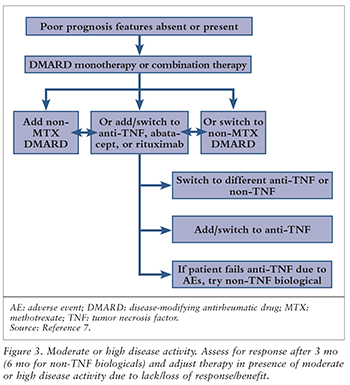
Tofacitinib may be used alone or in combination with other agents in a time frame similar to when anti-TNF therapy is being considered. The efficacy and safety of biologic use following failure of tofacitinib are unknown; however, a combination of other biologicals plus DMARDs may be used.7
Disease Response
Patients can be monitored for response and toxicity on a regular basis through standardized rating scales, laboratory data, and aggregate scoring systems. The ACR typically uses response levels of ACR20, ACR50, and ACR70, corresponding to 20%, 50%, and 70% improvement in disease level compared with baseline. Additionally, the DAS can incorporate the laboratory value of either ESR or CRP to monitor patient response. Finally, the Vectra DA test measures 12 biomarkers and uses an algorithm to assign a DAS that can be used to monitor patient response.9
The typical response to therapy is symptom improvement of 20% to 50%.1 Unfortunately, this may not be optimal for some patients, rendering it necessary to intensify therapy. Monotherapy is indicated for early RA with low disease activity; combination therapy is more appropriate for moderate-to-severe RA and for established RA.
Special Populations
Hepatitis: The new guidelines recommend etanercept for RA patients with hepatitis C. Biologicals are not recommended for patients with untreated chronic hepatitis B, owing to contraindications or intolerable AEs, or for those with treated chronic hepatitis B of Child-Pugh class B and higher. Patients should be screened for hepatitis B prior to starting biologicals. There are no recommendations regarding hepatitis A.7
Malignancy: Treatment is governed by how long ago the patient was treated for malignancy. Patients with a solid malignancy or nonmelanoma skin cancer treated >5 years ago should start or resume any biologic agent they would otherwise be qualified for. Patients with malignancy treated within the past 5 years are typically excluded from clinical trials, so there is a lack of knowledge in this subgroup; it is recommended to start or resume rituximab in these patients.7
Congestive Heart Failure: Anti-TNF biologicals should be avoided in patients with New York Heart Association Class III or IV congestive heart failure and ejection fraction ≤50%.7
Tuberculosis (TB): Prior to starting biologicals, all patients should be screened for latent TB infection (LTBI) via the tuberculin skin test (TST) or interferon-gamma release assay (IGRA). If a patient previously received bacille Calmette-Guérin vaccine, the IGRA method is recommended because of the high rate of false-positives associated with the TST. If a patient is diagnosed with active TB, appropriate TB treatment should be completed before starting or resuming the biological. If latent TB is diagnosed, ≥1 month of TB treatment should be completed before the biological is started or resumed.7
If a patient tests positive for LTBI at baseline, clinical signs and symptoms of recurrence should be monitored, as these tests can remain positive even after successful treatment. LTBI testing should occur annually in RA patients who live, travel, or work where TB exposure is likely. In cases in which a patient develops TB and is successfully treated, the decision to restart biologic therapy must carefully weigh risk of recurrent infection against risk of disease progression and potential morbidity related to disease progression.7
Vaccinations: Immunizations are very important in the management of RA. Owing to increased awareness of the risks associated with preventable diseases such as influenza and pneumonia, especially in the elderly, the updated guidelines now include CDC-concordant vaccination recommendations.7
Before an RA patient begins treatment with a DMARD or biologic agent, it is recommended that all killed (pneumococcal, influenza IM, and hepatitis B), recombinant (human papillomavirus vaccine for cervical cancer), and live attenuated (herpes zoster) vaccinations be administered. Even if an RA patient has already begun therapy, administration of these vaccines is still beneficial and recommended. The only exception is patients already taking biologicals: The live attenuated herpes zoster vaccination should not be administered to these patients.
Emerging Therapies
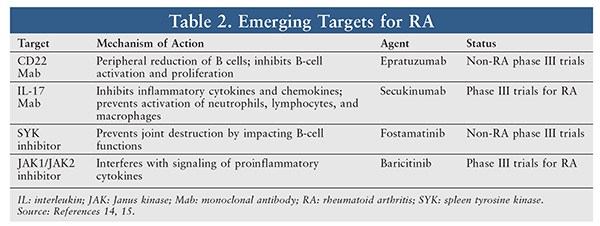
Despite the success of modern targeted therapy, many patients have exhausted all available treatments, and there is considerable need for new therapies. Emerging therapies target the cellular and molecular stages of RA: initial activation of the immune system leading to the inflammatory cascade (initiation phase), establishment of chronic pathology (chronification) with perpetuation of inflammation in joints and extra-articular sites (transformation phase), and irreversible organ damage resulting from destruction of the target tissues (effector phase).14,15
Several targets are currently under investigation (TABLE 2) in clinical trials. Some emerging therapies target and deplete B and T cells directly by inhibiting B-lymphocyte antigen and CAMPATH-1 antigen clusters of differentiation proteins. Other trials target IL cytokines, which are important in the recruitment of monocytes and neutrophils to the site of inflammation. Finally, spleen tyrosine kinase and JAK targets, which interfere with the signaling of various proinflammatory cytokines, and granulocyte-macrophage colony-stimulating factor are also targets of investigational therapy.14,15
Pharmacist’s Role
Because prompt diagnosis and treatment initiation are crucial to slowing disease progression, patient access allows pharmacists to play an important role in early detection of undiagnosed RA and avoidance of potentially significant medical-resource use and costs. Pharmacists contribute considerably to ensuring optimal selection of therapy based on factors such as cost-benefit ratio, dosing frequency, route of administration, patient vaccination status, and considerations for special populations. Furthermore, pharmacists can increase medication adherence through patient education and follow-up to ensure monitoring of side effects and symptom improvement with current therapy. Pharmacist intervention in RA management has been proven to optimize economic and patient outcomes.16 Considering the patient’s education needs, the specialized character of the medications available, and the high-risk nature of the AE profile, RA patients are an ideal population for pharmacist outreach with medication therapy management, vaccinations, and other innovative programs.
Conclusion
RA is a disease state that requires early intervention to prevent long-term complications and morbidity. Patients will benefit from early therapy with DMARDs that slow disease progression. Unfortunately, the most effective medications have numerous and troublesome side effects, in particular immunosuppression, leading to infection and reactivation of latent infections. The development of additional therapeutic options with novel mechanisms will be necessary to achieve higher response rates.
REFERENCES
1. Silman AJ, Hochberg MC, eds. Epidemiology of the Rheumatic Diseases. 2nd ed. New York, NY: Oxford University Press; 2001.
2. Furneri G, Mantovani LG, Belisari A, et al. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol. 2012;30(4 suppl 73):S72-S84.
3. Yelin E, Cisternas M, Foreman A, et al. National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions—United States, 2003. MMWR. 2007;56:4-7.
4. National Collaborating Centre for Chronic Conditions (UK). Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. London, England: Royal College of Physicians; 2009.
5. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205-2219.
6. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907-916.
7. Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625-639.
8. Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012;64:640-647.
9. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492-509.
10. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762-784.
11. Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2006;63:2451-2465.
12. DynaMed [online subscription database]. https://dynamed.ebscohost.com. Accessed March 31, 2014.
13. Consumer Reports Best Buy Drugs. Using biologic drugs to treat rheumatoid arthritis: comparing effectiveness, safety, side effects, and price. www.consumerreports.org/cro/2013/03/using-biologic-drugs-to-treat-rheumatoid-arthritis/index.htm. Accessed August 13, 2014.
14. Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:77-88.
15. ClinicalTrials.gov. RA studies. www.clinicaltrials.gov. Accessed February 28, 2014.
6. Barlow JF, Faris FJ, Wang W, et al. Impact of specialty pharmacy on treatment costs for rheumatoid arthritis. Am J Pharm Benefits. 2012:4(special issue):SP49-SP56.
To comment on this article, contact rdavidson@uspharmacist.com.






