US Pharm. 2015;40(11)(Specialty&Oncology suppl):7-10.
ABSTRACT: In patients with cancer, many factors increase the risk of developing venous thromboembolism (VTE) and recurrent VTE. Additionally, many potentially complicating clinical factors must be considered in selecting a treatment for VTE. Current guidelines recommend low-molecular-weight heparin (LMWH) to treat VTE in cancer patients; warfarin is a second-line option. Direct-acting oral anticoagulants (DOACs), which are newer agents for VTE treatment, may be a useful option in patients with cancer. Clinical trials comparing DOACs with warfarin have been conducted, but until LMWH and DOACs are compared, LMWH is preferred for treating VTE in cancer patients.
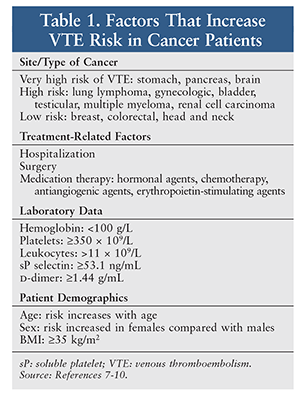
In patients with cancer, venous thromboembolism (VTE) has been associated with a poor prognosis and is the second leading cause of death.1-4 Cancer patients have a sevenfold increase in risk of VTE overall, with an increase of up to 28-fold in certain types of cancer.5 Additionally, cancer patients who develop VTE and patients who have VTE upon cancer diagnosis are much more likely to develop recurrent VTE.6 Khorana and colleagues developed a risk-assessment tool for chemotherapy-associated thrombosis in cancer patients that includes site of malignancy; prechemotherapy platelet, hemoglobin, and leukocyte counts; use of RBC growth factors; and BMI.7 However, many factors can increase the risk of VTE (TABLE 1).7-10 Not only do cancer patients have an elevated risk of developing VTE, recurrent VTE, and bleeding complications, but treatment-related factors such as nausea, vomiting, poor appetite, and renal and hepatic dysfunction can further complicate the treatment of VTE.
Tumors have several biologic mechanisms that can lead to increased coagulopathy in cancer.10-12 Falanga and colleagues list the principal mechanisms of thrombosis from tumor cells (and sometimes host cells) as follows: expression of hemostatic proteins by tumor cells, especially tumor factor; production of microparticles; production of inflammatory cytokines, including tumor necrosis factor-alpha and interleukin-1 beta; production of proangiogenic factors, such as vascular endothelial growth factor and basic fibroblast growth factor; and expression of adhesion molecules.10 Many of these mechanisms also contribute to tumor growth and metastasis.10,11
Treatment Guidelines
The greatest amount of data on the treatment of VTE in cancer patients involves vitamin K antagonists (VKAs) and low-molecular-weight heparin (LMWH), specifically enoxaparin and dalteparin. Based on lower rates of VTE recurrence, guidelines for prevention and treatment of VTE in cancer patients prefer LMWH over VKAs; however, both classes are frequently used in this population.13-15 These agents are generally well tolerated, but patients may be undergoing treatment (including surgery, radiation, and antineoplastic agents, alone or in combination), which may increase the potential for adverse effects of anticoagulation. Antineoplastic agents can cause nausea and decreased appetite, which often result in a decreased and inconsistent dietary intake. They can also lead to renal and/or hepatic damage and dysfunction. Some patients cannot self-administer the SC injections required for LMWH, and others are dissatisfied with the increased injection burden associated with LMWH, which may lead them to refuse treatment. Additionally, cancer patients receiving anticoagulant therapy for VTE are at greater risk for serious bleeding events.6,12
Initial therapy for VTE in cancer patients may consist of LMWH in place of unfractionated heparin (UFH). The recommended use of LMWH as initial therapy comes from small clinical trials. A Cochrane Collaboration review analyzed 11 randomized, controlled trials comparing LMWH and UFH as initial therapy for VTE.16 This review demonstrated a statistically significant reduction in mortality at 3 months in patients receiving LMWH versus those receiving UFH (relative risk [RR] 0.71, 95% CI 0.52-0.98); however, a meta-analysis of three trials demonstrated no statistically significant reduction in recurrent VTE with LMWH compared with UFH (RR 0.78, 95% CI 0.29-2.08).16 A different Cochrane review assessed long-term treatment of VTE in seven randomized, controlled trials comparing LWMH with VKA.17 No statistically significant survival benefit (hazard ratio [HR] 0.96, 95% CI 0.81-1.14) was found, but there was a significant reduction in VTE recurrence (HR 0.47; 95% CI 0.32-0.71).17
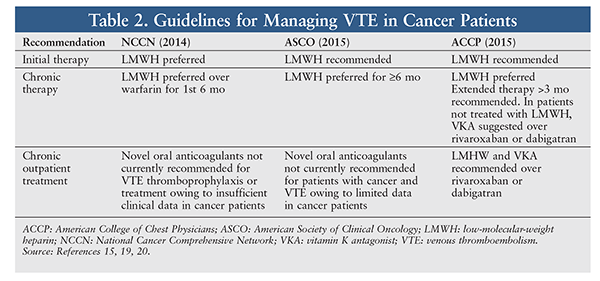
The largest trial to assess options for VTE treatment in cancer patients was the open-label, multicenter CLOT trial, in which subjects were randomized to dalteparin or warfarin for 6 months.18 In total, 27 of 336 subjects (8%) receiving dalteparin and 53 of 336 (15.8%) receiving warfarin had recurrent VTE (HR 0.48; P = .002). At 6 months, there were no significant differences in mortality (39% for dalteparin vs. 41% for warfarin) or major bleeding (6% for dalteparin vs. 4% for warfarin). The CLOT trial provided evidence that dalteparin was preferable to warfarin to reduce recurrent VTE in cancer patients and supported the recommended use of LMWH for VTE in cancer patients. TABLE 2 gives an overview of guidelines on managing VTE.15,19,20
Direct-Acting Oral Anticoagulants
Direct-acting oral anticoagulants (DOACs) are newer agents that have potential for treating VTE in patients with cancer. This section will discuss comparisons of DOACs and VKAs from clinical trials with subgroup analyses of use for VTE treatment in cancer patients.
DOACs comprise two new classes of medications: direct thrombin inhibitors and factor Xa inhibitors. The agents, in order of FDA approval for VTE, are rivaroxaban, dabigatran, apixaban, and edoxaban. These agents vary in terms of mechanism of action, pharmacokinetics, dosing, and bleeding risk.21-24
Mechanisms of Action: Warfarin inhibits the synthesis of vitamin K–dependent clotting, which involves factors II, VII, IX, and X, and the anticoagulant proteins C and S.25 Rivaroxaban, apixaban, and edoxaban are selective factor Xa inhibitors that do not require a cofactor such as antithrombin III.21,23,24 By directly blocking thrombin, dabigatran inhibits the thrombin-dependent conversion of fibrinogen to fibrin.22 Dabigatran and the factor Xa inhibitors indirectly inhibit thrombin-induced platelet aggregation and inhibit both free and clot-bound fibrin.21-24 UFH and LMWH cannot inhibit fibrin-bound thrombin, which theoretically may be a disadvantage because bound thrombin can continue to promote thrombus development.26
Pharmacokinetics: DOACs exhibit a more rapid onset of action and a shorter half-life than warfarin since warfarin is not active against clotting factors that are in circulation at the time of initiation.21-24 Except for dabigatran, oral anticoagulants are metabolized to varying degrees by CYP450 enzymes (TABLE 3).21-24,27-31 Warfarin is eliminated through hepatic metabolism, whereas DOACs have some degree of renal clearance.21-25
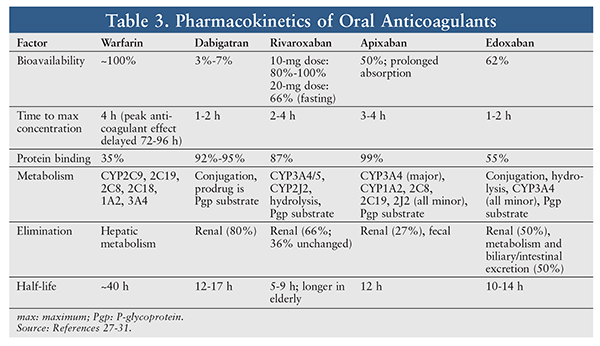
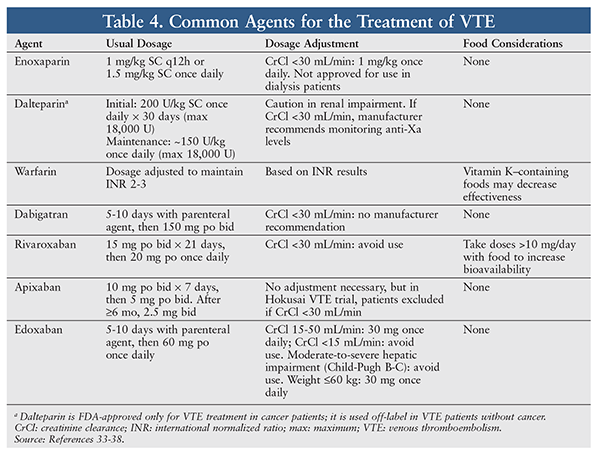
Dosing and Dosage Adjustments: Dosing and the number of administrations needed per day vary between oral anticoagulants. Some agents have dosing variations and different dosage-adjustment recommendations based on the indication, and all agents require adjustments in certain circumstances.27-30,32 Dabigatran, rivaroxaban, and edoxaban should be avoided in patients with end-stage renal disease (ESRD).27,28,32 According to labeling information, apixaban is an option in patients with ESRD; however, subjects (dialysis or nondialysis) with a creatinine clearance <15 mL/min were excluded in key efficacy and safety studies.29
Warfarin, which has numerous food and drug interactions, requires laboratory monitoring.30 The lack of routine monitoring, absence of food interactions, and occurrence of fewer drug interactions are important advantages of DOACs. Rivaroxaban is the only DOAC that has a food consideration: Doses >10 mg daily should be taken with food to increase bioavailability.28 All DOACs are P-glycoprotein (Pgp) substrates and therefore have dosing recommendations if a Pgp inducer or inhibitor is being used concomitantly.27-29,32 Rivaroxaban and apixaban are significantly metabolized by CYP3A4, so drug interactions may occur with medications that inhibit or induce this enzyme.28,29 Another important difference in dosing is that, when used for the treatment of VTE, both dabigatran and edoxaban require initial administration of a parenteral agent.27,32 Rivaroxaban and apixaban were studied and approved for use as initial treatment.28,29 TABLE 4 summarizes dosing, dosage-adjustment recommendations, and food considerations for DOACs.33-38
Adverse-Effect Profiles: Compared with warfarin and anti-Xa agents, dabigatran has a higher incidence of gastrointestinal (GI) adverse effects such as dyspepsia, upper abdominal pain, and gastritis-like symptoms.39-44 However, as with all oral anticoagulants, bleeding is the most common and significant adverse effect. In VTE studies, major bleeding rates, as compared with those of warfarin, were similar for dabigatran, lower for apixaban, and lower for rivaroxaban and edoxaban.39-44 DOACs had a similar or lower rate of intracranial bleeds than warfarin and did not have a higher incidence of fatal bleeding; dabigatran had more frequent GI bleeds and apixaban had fewer.39-44
Clinical Trials: No prospective, randomized trials have compared any DOAC to warfarin or LMWH for VTE treatment in patients with cancer. However, subset analyses of DOAC trials in patients with VTE have been conducted. TABLE 5 (see www.uspharmacist.com) summarizes DOAC subset analyses of VTE treatment in cancer patients.5 Several important observations can be made:
• Rates of recurrent VTE and bleeding are increased in cancer patients compared with patients without cancer.
• Rates of recurrent VTE in cancer patients are lower in all DOAC groups compared with warfarin.
• Rates of bleeding in cancer patients are numerically lower in all DOAC groups compared with warfarin (statistically significantly lower with rivaroxaban).
• The largest subset (EINSTEIN-DVT & EINSTEIN PE) was comparable in size to the CLOT trial.
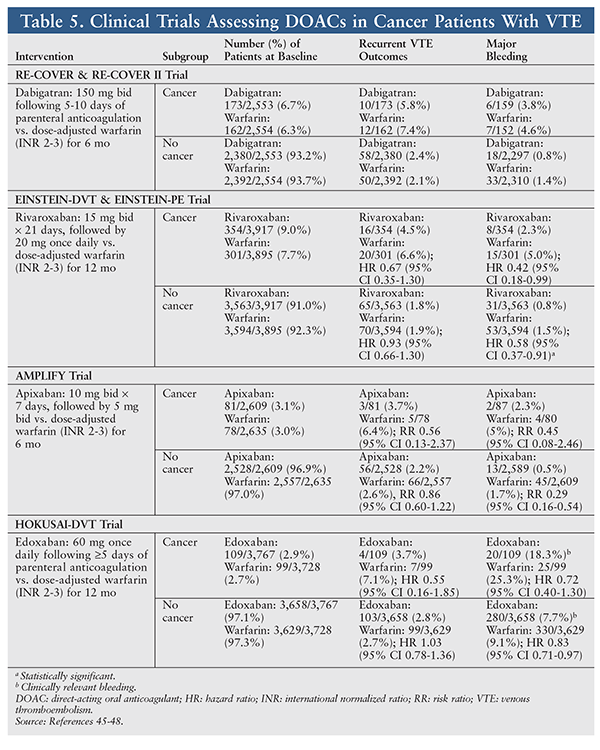
A six-study meta-analysis by Vedovati and colleagues assessed the efficacy and safety of DOACs in patients with VTE and cancer.20 This meta-analysis found a recurrent VTE rate of 3.9% in patients receiving DOACs versus 6.0% in warfarin patients (odds ratio [OR] 0.63, 95% CI 0.37-1.10). Major bleeding was lower with DOACs than with warfarin (3.2% vs. 4.2%; OR 0.77, 95% CI 0.41-1.44). It was concluded that DOACs may be as effective and safe as warfarin in patients with cancer and VTE.
It is important to note that these were subgroup analyses of larger clinical trials, and none of the DOACs is indicated for use in patients with cancer. There are several limitations, including a lack of details on patient characteristics such as timing of cancer diagnosis, type and stage of cancer, and concomitant use of medications that may be associated with increased risk of thromboembolism. In addition, these trials compared DOACs with warfarin but not with LMWH, which is recommended in guidelines. Future trials should assess the efficacy and safety of DOACs compared with LMWH.
Conclusion
Pharmacists can play an important part in ensuring that patients with cancer receive the most appropriate anticoagulation for VTE based on guideline recommendations, additional available data, and patient-specific factors to prevent recurrent VTE and major bleeding. There are many factors to consider in selecting a therapy for this patient population, and pharmacists are optimally positioned to help prevent adverse effects of anticoagulation and bleeding by educating patients and ensuring appropriate dosing and monitoring throughout the course of therapy. Although LMWH and warfarin remain the standard of care, DOACs show promise for the treatment of VTE in cancer patients and may be appropriate for a segment of this population. However, until DOACs and LMWH are compared directly, LMWH will continue to be preferred for VTE treatment in cancer patients.
REFERENCES
1. Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339-2346.
2. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846-1850.
3. Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125:490-493.
4. Deng A, Galanis T, Graham MG. Venous thromboembolism in cancer patients. Hosp Pract (1995). 2014;42:24-33.
5. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715-722.
6. Chee CE, Ashrani AA, Marks RS, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood. 2014;123:3972-3978.
7. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
8. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
9. Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer—a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404-1413.
10. Falanga A, Russo L, Milesi V. The coagulopathy of cancer. Curr Opin Hematol. 2014;21:423-429.
11. Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res. 2015;135(suppl 1):S8-S11.
12. Rodrigues CA, Ferrarotto R, Kalil Filho R, et al. Venous thromboembolism and cancer: a systematic review. J Thromb Thrombolysis. 2010;30:67-78.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Cancer-associated venous thromboembolic disease. Version 1.2015. www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed September 30, 2015.
14. Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11:56-70.
15. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654-656.
16. Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011;(6):CD006649.
17. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
18. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.
19. Zytiga (abiraterone) product information. Horsham, PA: Janssen Biotech, Inc; May 2015.
20. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147:475-483.
21. Savaysa (edoxaban) product information. Parsippany, NJ: Daiichi Sankyo, Inc; September 2015.
22. Pradaxa (dabigatran) product information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; January 2015.
23. Xarelto (rivaroxaban) product information. Titusville, NJ: Janssen Pharmaceuticals, Inc; September 2015.
24. Eliquis (apixaban) product information. Princeton, NJ, and New York, NY: Bristol-Myers Squibb Co and Pfizer Inc; June 2015.
25. Coumadin (warfarin) product information. Princeton, NJ: Bristol-Myers Squibb Co; October 2011.
26. Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. 2011;123:1436-1450.
27. Eisen T, Sternberg CN, Robert C, et al. Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104:93-113.
28. Martins F, de Oliveira MA, Wang Q, et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013;49:293-298.
29. de Oliveira MA, Martins E, Martins F, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47:98-1003.
30. Pilotte AP, Hohos MB, Polson KM, et al. Managing stomatitis in patients treated with Mammalian target of rapamycin inhibitors. Clin J Oncol Nurs. 2011;15:E83-E89.
31. Afinitor (everolimus) product information. East Hanover, NJ: Novartis Pharmaceuticals Corp; January 2015.
32. Institute for Safe Medication Practices. ISMP list of high-alert medications in community/ambulatory healthcare. http://ismp.org/communityRx/tools/highAlert-community.pdf. Accessed September 30, 2015.
33. Carey ET, Forrey R, Haughs D, et al. A second look at utilization of a closed-system transfer device (PhaSeal). Am J Pharm Benefits. 2011;3:311-318.
34. Wong SF Bounthavong M, Nguyen C, et al. Implementation and preliminary outcomes of a comprehensive oral chemotherapy management clinic. Am J Health Syst Pharm. 2014;71:960-965.
35. Xeloda (capecitabine) product information. South San Francisco, CA: Genentech USA, Inc; March 2015.
36. Funakoshi T, Latif A, Galsky MD. Safety and efficacy of addition of VEGFR and EGFR-family oral small-molecule tyrosine kinase inhibitors to cytotoxic chemotherapy in solid cancers: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev. 2014;40:636-647.
37. Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: definitions and clinical implications. Cancer. 2011;117:688-697.
38. Wolter P, Schöffski P. Targeted therapies in the treatment of GIST: adverse events and maximising the benefits of sunitinib through proactive therapy management. Acta Oncol. 2010;49:13-23.
39. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764-772.
40. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. New Engl J Med. 2009;361:2342-2352.
41. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
42. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
43. Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406-1415.
44. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
45. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114:150-157.
46. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematology. 2014;1:e37-e46.
47. Agnelli G, Buller HR, Cohen A, et al. Apixaban for the treatment of venous thromboembolism in cancer patients: data from the AMPLIFY trial. Poster presented at: European Society of Cardiology Congress 2014; Barcelona, Spain.
48. Raskob GE, Buller H, Angchaisuksiri P, et al. Edoxaban for long-term treatment of venous thromboembolism in cancer patients [abstract 211]. Blood. 2013;122(21):epub.
To comment on this article, contact rdavidson@uspharmacist.com.





