US Pharm. 2010;35(6):36-37.
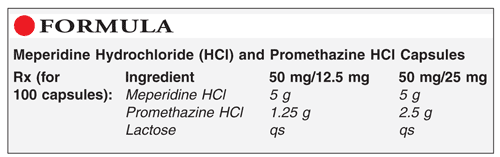
Method of Preparation: Note: It is necessary to determine the quantity of lactosee required as a filler for the capsules prior to compounding this preparation.1-3
Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Pulverize the powders to approximately the same particle size. Mix together the meperidine hydrochloride (HCl) and promethazine HCl. Blend in the lactose geometrically; mix until uniform. Encapsulate into the desired number of capsules. Clean, package, and label.
Use: Meperidine HCl and promethazine HCl capsules have been used in the treatment of moderate-to-severe pain.
Packaging: Package in tight, light-resistant containers.4
Labeling: Keep out of the reach of children. Use only as directed.
Stability: A beyond-use date of up to 6 months is appropriate for this preparation.4
Quality Control: Quality-control assessment can include weight–overall average weight; weight–individual weight variation; dissolution of capsule shell; disintegration of capsule content; active-drug assay results; physical appearance (color, uniformity, extent of fill, locked); and physical stability (discoloration, changes).5
Discussion: Thousands of drug products have been removed from the market for economic reasons, as well as for safety and efficacy. Removal for economic reasons may be due to inadequate sales or the introduction of competing products; it also frequently occurs when companies merge and the slower-selling products are removed from the market. When the latter occurs, if another pharmaceutical company does not make arrangements for marketing, the most common method for physicians to continue to prescribe the drug(s) for their patients is through compounding. In general, the formulations for these discontinued products are available in reference books and on Web sites.6
Until about 1976, meperidine HCl and promethazine HCl capsules (Mepergan Capsules) were marketed by Wyeth. The combination uses the synergistic effects of the analgesic meperidine HCl and the antihistamine promethazine HCl. This combination was available in a regular strength and a stronger combination (Mepergan Fortis). The combination was available in both oral and parenteral dosage forms.6 An advantage to the combinations given here is that additional active ingredients may easily be added to the formula.
Meperidine HCl (C15H21NO2.HCl, MW 283.79, isonipecaine HCl, pethidine HCl) occurs as a fine, white, crystalline, odorless powder. It is highly soluble in water and soluble in alcohol. It melts at about 186°C to 189°C and should be preserved in well-closed, light-resistant containers. A solution of 5 g in 100 mL purified water has a pH of about 5.0. Meperidine HCl is used to manage moderate-to-severe pain and as an adjunct to anesthesia and preoperative sedation. It is available in injection and tablet dosage forms.4
Promethazine HCl (C17H20N2S.HCl, MW 320.88, Phenergan) is a phenothiazine derivative with potent first-generation antihistaminic and antiemetic properties and a prolonged duration of action. It has been combined with codeine, dextromethorphan, and meperidine to utilize their synergistic effects in the treatment of moderate-to-severe pain. It occurs as a white to faint yellow, crystalline, practically odorless powder. It slowly oxidizes and acquires a blue color on prolonged exposure to air. It is freely soluble in water, hot dehydrated alcohol, and chloroform. It should be protected from light. Promethazine is available as injection, suppositories, syrup, and tablets.4 The injection form has a pH of 4.0 to 5.5.
Lactose (C12H22O11, milk sugar, saccharum lactis) is available either in anhydrous form or as a monohydrate. Commercially, it is available as anhydrous alpha-lactose, alpha-lactose monohydrate, and anhydrous beta-lactose, and with different grades and particle characteristics. It is widely used as a diluent in numerous dosage forms, and in lyophilized products and infant formulas. It is soluble in water to the extent of about 1 g in 4.63 mL, but is practically insoluble in ethanol. A 9.75% solution is iso-osmotic with serum. If lactose is stored under conditions of greater than 80% humidity, mold growth may occur. Lactose will exhibit a Maillard-type reaction (brownish discoloration) when heated with compounds containing a primary amine group. This reaction occurs more rapidly under alkaline conditions. Lactose is listed as incompatible with amino acids, aminophylline, and amphetamines (owing to the amine groups present).7
REFERENCES
1. Allen LV Jr. The basics of compounding: compounding powder-filled capsules. IJPC. 1999;3:209-215.
2. Glasnapp A, Hua YT. Making capsules using a solid oral dosage form of a different strength. IJPC. 1998;2:240.
3. Allen LV Jr. Standard operating procedure: developing a capsule formulation. IJPC. 2001;5:392-393.
4. USP Pharmacists’ Pharmacopeia. 2nd ed. Rockville, MD: US Pharmacopeial Convention, Inc; 2008;219-220,775-779,1444.
5. Allen Jr LV. Standard operating procedure for performing physical quality assessment of powder-filled, hard-gelatin capsules. IJPC. 1999;3:232-233.
6. CompoundingToday.com. Drug information: discontinued medications. www.compoundingtoday.com [subscription required]. Accessed May 25, 2010.
7. Edge S, Kibbe AH, Shur J. Lactose (anhydrous and monohydrate). In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:359-361,364-369.
To comment on this article, contact rdavidson@uspharmacist.com.





