US Pharm.
2008;33(6)(Generic Drug Review suppl):10-17.
Pharmaceuticals are a large
part of health care expenditures in the United States. According to the
Generic Pharmaceutical Association, 286.5 billion dollars were spent on
medications in the U.S. in 2007.1 Prescription medications
dispensed in community pharmacies comprised 10.1% of health care expenditures.
This is an increase of 6% from 1995.2 Interestingly, the growth in
drug expenditures has plateaued over the past few years. Prescription drugs
accounted for 9.9% of national health expenditures in 2002, increasing by only
0.2% in 2005.2 One of the reasons for this slowdown is the use of
generic drugs.3
About 65% of all prescriptions
are filled with generic drugs, yet they generics account for only 20% of total
prescription costs.1 The FDA calculated some eye-opening figures
using data from the 2004 IMS Health's National Prescription Audit Plus. If
patients have their prescriptions filled generically, drug costs can fall by
14% to 16% per day, depending on the condition being treated. Those patients
fortunate enough to be able to fill all of their prescriptions generically
could expect a 52% decrease in the daily costs of their medications.4
On average, generic medications cost from 30% to 80% less than the brand drug.
1 Generic manufacturers do not have to carry the expense of research and
development or the marketing of their products compared to brand
manufacturers. This enables the generic manufacturers to sell the same drug at
a fraction of the cost. This cost differential is a large part of the
ever-increasing presence of generics in the pharmacy industry. With the
filling of generic prescriptions on the rise, it is important that pharmacists
know the approval process that the FDA requires to bring a generic equivalent
to the market.
Drug Approval Process
The drug approval
process is vastly different between the innovator product and the generic
equivalent. The brand manufacturers must go through a tedious and
time-consuming process in order to get their drug approved. There are several
phases involved in the approval of innovator drugs, starting with the
preclinical phase.
Preclinical Phase:
In the preclinical phase, all testing is performed on laboratory animals. The
sponsor of the drug entity determines the toxic and pharmacologic effects
using in vitro and in vivo testing. Absorption, metabolism, and the rate of
excretion of the parent drug and the metabolites are investigated during the
preclinical phase. The sponsor also looks for the toxicity of the metabolites.
After the successful completion of the preclinical phase, an Investigational
New Drug (IND) Application is filed with the FDA.
Phase I:
During phase I, the IND is typically tested on healthy humans. Patients may
be included in phase I trials, but that is not the norm. Phase I trials
consist of approximately 20 to 80 subjects and are used to determine the
metabolic and pharmacologic actions of the drug in humans. The investigators
assess the safety and incidence of adverse effects as well as the drug
metabolism, structureñactivity relationships, and mechanism of action.
Phase II:
Phase II trials are conducted on several hundred patients and are designed to
gather preliminary data on the effectiveness of the drug. During phase II,
common side effects and risks in patients are determined.
Phase III:
Phase III trials are much larger and consist of several hundred to several
thousand subjects. Phase III studies consist of both controlled and
uncontrolled trials that investigate additional safety and efficacy
information. The information gathered in phase III trials is used to create
the package insert.
After the sponsor successfully
completes all three phases of human trials, they submit a New Drug Application
(NDA) with the FDA.5
Abbreviated New Drug
Applications
The approval
process for generic substitutes is far simpler, but it has not always been
that way. In 1970 the FDA introduced the Abbreviated New Drug Application
(ANDA). At that time, the ANDA could only be used to approve the generic
versions of drugs that had been approved between 1938 and 1962.6
The generic manufacturers seeking approval for a generic equivalent of a brand
name drug approved after 1962 had to conduct their own studies establishing
safety and efficacy.6 These requirements served as a barrier for
the development of cheaper generic versions of innovator drugs. The FDA's
stringent approval process remained in effect until 1978, after which
manufacturers only had to cite published reports of trials that documented the
safety and efficacy of the drug entity in order to obtain approval.
This became problematic, however, because many of the trials required to
satisfy FDA requirements were not published.6
The Drug Price Competition
and Patent Term Restoration Act
In 1984, the Drug
Price Competition and Patent Term Restoration Act, also called the
Hatch-Waxman Act, was passed. This important legislation affected the generic
industry profoundly. This amendment to the Federal Food, Drug, and Cosmetic
Act allowed for the use of an ANDA for approval of a "new drug." The ANDA had
to include "1) information to show that the conditions of use prescribed in
the labeling proposed for a new drug have been previously approved for a drug
that appears on a list prepared by the Secretary of Health and Human Services
(listed drug); and 2) a certification relating to such patents covering such
listed drug."7 In other words,† no preclinical or clinical
studies need to be done to demonstrate safety and efficacy.6 The
Hatch-Waxman Act allows brand manufacturers a five-year patent extension in
order to recoup their time lost in the approval process. This is allowed if
the patent has not yet expired when the application for extension is submitted
and the patent has never been extended before. This extension request must be
submitted by "the owner of record of the patent or its agent."7
The act promoted research and development of new drug entities and encouraged
generic manufacturers to produce equivalents by simplifying the approval
process. They only had to prove bioequivalence to the innovator drug.
Determining Bioequivalence
Generic drugs,
called pharmaceutical equivalents, must have the same active ingredient in the
same strength as the brand drug, must be in the same dosage form and be
administered in the same way as the innovator drug, and must be have labeling
essentially the same as that of the brand name drug. The FDA also requires the
generic drug's chemistry, manufacturing steps, and quality control measures to
be fully documented by the generic manufacturer. The FDA must be assured that
the raw materials and finished product meet U.S. Pharmacopoeia specifications
and that the potency of the equivalent will remain unchanged until the
expiration date on the label. Generic manufacturers need to comply with the
federal regulations of good manufacturing practices and include a full
description of the facilities used to manufacture, process, test, package, and
label their products.
Generic pharmaceutical
manufacturers must also prove bioequivalence. Bioequivalence means "the
absence of a significant difference in the rate and extent to which the active
ingredient or active moiety in pharmaceutical equivalentsÖ.becomes available
at the site of drug action when administered at the same molar dose under
similar conditions in an appropriately designed study."8,9 Two
drug products are deemed to be bioequivalent if it can be demonstrated that
they have an equivalent rate and extent of absorption. Based on these
premises, it can be inferred that if two drugs products contain chemically
identical drug substances and are delivered to the site of action at the same
rate and extent of each other, they are equivalent. They may then be
substituted for each other.10
Using a two-treatment
crossover design with 24 to 36 healthy adults in the fasting state, the
typical pharmacokinetic bioavailability or bioequivalence study is conducted.
11 A single dose of one of the drugs being compared is administered and
the blood or plasma concentrations are measured over time. The parameters used
to determine the rate and extension of absorption are the area under the
plasma concentrationñtime curve (AUC, determines extent of absorption) and the
maximum or peak drug concentrations (Cmax, determines rate of absorption).
10 After an elimination period of the drug, at least three times the
half-life of the active drug and/or its active metabolites measured in the
blood, the other drug substance is administered.11
Other study designs may be
appropriate when blood-plasma concentrations are not useful to determine the
delivery of the drug to the site of action. This would apply to inhalers,
nasal sprays, or dermatological topical formulations. In these cases, an in
vitro study or an equivalence study might be used to determine clinical or
pharmacodynamic endpoints.10
The data resulting from these
studies is statistically evaluated using the two one-sided tests procedure.
10 This is a test designed to determine equivalence with the assumption
is that there may be some inequivalence.12 The first of the two
one-sided tests determines if a generic product, when substituted with a brand
name product, exhibits significantly less bioavailability. The second of the
two one-sided tests determines whether a brand drug, when substituted by a
generic, exhibits significantly less bioavailabilty.10 A difference
of greater than 20% for each of the statistical tests is considered
significant and results in failure of bioequivalence between the two drugs.
This translates to an 80% difference in the bioavailability of the products.
All of the data is expressed as a ratio of average values of the AUC and Cmax.
Therefore, the limits of acceptable bioavailability for a pharmaceutical
equivalent are 80% and 125% (reciprocal of 80%) of the innovator product.
10 Even though the FDA contends that this process of approval is
adequate, there are others who do not agree.
Weaknesses in the FDA
Approval Process
There is some
question as to whether a bioequivalent generic drug will be clinically
comparable to the reference drug.9 Will the generic demonstrate the
same efficacy and tolerability as the original brand name drug?
The use of healthy volunteers
to determine bioequivalence is another suspect practice. The extrapolation of
bioequivalence demonstrated in normal healthy subjects to bioequivalence in a
patient population has been criticized.9 In order to ensure a
homogeneous subject population, many variables need to be controlled. The
study subjects are healthy males aged 18 to 55. They do not take any
concurrent medications, do not smoke, and are of normal body height and
weight. In addition, all subjects receive a controlled diet.9 This
is an excellent study design used to ensure that any bioinequivalence is due
to the formulation of the test drug and not because of any other differences
between subjects. However, there are differences in pharmacokinetic properties
of a drug if these variables are not so closely controlled. Concurrent
diseases, concurrent medications, diet, differences in first-pass metabolism,
and the presence of food in the stomach can all affect the way a drug is
absorbed, metabolized, and excreted. Some gastrointestinal factors that may
alter the pharmacokinetic parameters include gastric pH, blood flow, and
bacterial flora.9 Some researchers believe these variables should
be taken into consideration. There are some problems with the use of patients
in bioequivalence studies.
There is also an ethical issue
to be considered if patients are used as test subjects. If the generic drug
proves to be substandard, it may result in toxicity or worsening of the
disease state in the patient. Another reason to avoid using patients as study
subjects is the inherent variability in a heterogeneous sample. This would
require larger patient populations, which may result in longer study times at
a greater expense. Finally, there may be differences in the drug response
and/or pharmacokinetic parameters, which are simply the result of a
progressing disease process and not a problem in the formulation.6
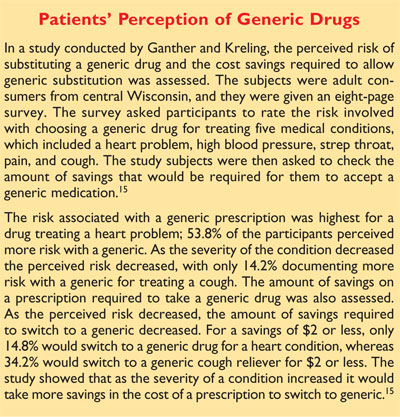
The use of single-dose studies is
another area of concern. The FDA states that the use of single-dose studies is
generally more sensitive in assessing the release of an active metabolite from
the parent drug into the systemic circulation. The problems with single-dose
studies include the fact that most drugs are not given as a single dose and
must be maintained at a steady state in order to obtain the therapeutic
effect. Often the drug concentration at steady state is higher than that after
a single dose. It is also thought that some of the inert ingredients in the
generic formulation may affect the pharmacokinetics at steady state, which may
not be noticed after a single dose.9
The use of the same regulatory
limit (80% to 125%) for all medications, including narrow therapeutic index
(NTI) drugs, has been under scrutiny. Some believe the bioequivalence
requirements should be more stringent when evaluating NTIs.9
There has been concern about
drug imports. Baxter's heparin recall brings this issue to the forefront again.
13 On December 11, 2007, an agreement was reached between the U.S.
Department of Health and Human Services and the State Food and Drug
Administration (SFDA) of the People's Republic of China. In general, this
agreement serves to establish a consensus with regard to the exportation of
drugs, excipients, and medical devices from China into the U.S. and engage in
information sharing in order to better understand each party's regulation
process. This will result in improvement of the authenticity, quality, safety,
and efficacy of drugs, excipients, and medical devices imported from China.
14 Any firms that import drugs to the U.S. will need to be registered by
the SFDA. These firms will need to be certified by the SFDA that they meet FDA
requirements for drug importation into the U.S
The Orange Book
The Orange
Book is a publication listing approved prescription drug products with
therapeutic equivalence evaluations.10 It is published
electronically (as well as in print) and can be found at www.fda.gov/cder/ob/.
The Orange Book is an excellent reference tool for pharmacists
practicing drug product selection. It lists evaluation codes identifying which
products have been tested and deemed to be bioequivalent to the innovator
product. The electronic version has a query field that may be searched by
active ingredient, proprietary name, applicant holder, application number, and
patent.10 This enables quick and easy retrieval of generic
equivalents for a brand name drug.
The equivalence codes assigned
to the generic products begin with either an A or a B. Those products
designated A products have documented or potential problems resolved with in
vivo or in vitro testing as appropriate. B products are those that have not
had any actual or potential bioequivalence problems resolved. There are three
main policies into which a B product may fall: 1) the drug products have
either active ingredients or are manufactured in dosage forms that have had
documented actual or potential bioequivalence problems and for which there has
not been adequate evidence submitted to the FDA resolving such issues; 2) the
quality standards are inadequate or there is not enough sufficient evidence
for which to determine bioequivalence; and 3) the drugs are under regulatory
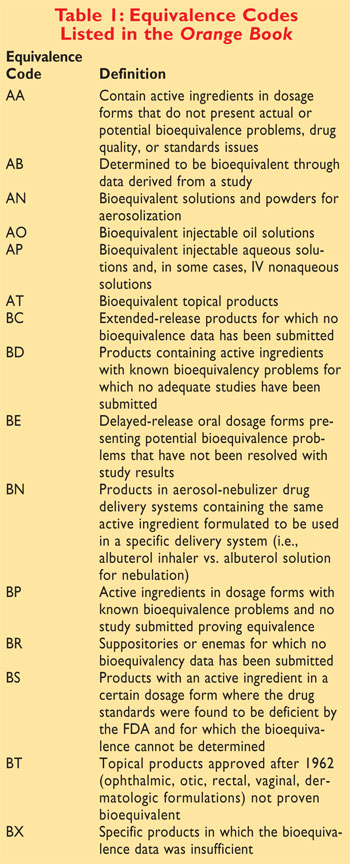
review.10 Please refer to TABLE 1 for the equivalence codes
listed in the Orange Book.
There are some special situations that require
more explanation. In the case of amino acid and protein hydrolysate
injections, equivalency falls in a gray area. There are different types and
amounts of amino acids in each; therefore, they are not equivalent. It needs
to be pointed out, though, that when nitrogen balance is the only therapeutic
objective and different amino acid content is not important, products can be
considered equivalent if they have the same total amount of nitrogen.10
Another special situation exists with Follitropin formulations. Based on data
derived from physiochemical tests and bioassay, Follitropin Alfa and
Follitropin Beta are not distinguishable from each other.10
There are multiple reference-listed drugs
(those reference drugs generic products are tested against) for levothyroxine
sodium. Some of these reference-listed drugs were approved by demonstrating
bioequivalence to other reference listed drugs. This has resulted in the FDA
not granting a blanket equivalence rating for all of the formulations. They
have created a chart showing which brand drugs are equivalent to each other.
For instance, the Mylan brand of levothyroxine sodium is equivalent to the
corresponding strengths of Unithroid.10
Sometimes, an equivalence code contains a
number at the end (AB1, AB2Ö). This is done when there are two or more
reference-listed drugs with the same active ingredient in the same strength
but which are not bioequivalent. For instance, Procardia XL and Adalat CC are
listed under the active ingredient nifedipine, yet they are not bioequivalent
to each other. Therefore, generic products that are equivalent to Adalat CC
are given an AB1 rating and those equivalent to Procardia XL are given an AB2
rating.10 It is important to remember that most states have a
formulary dictating which products may be substituted
Narrow Therapeutic Index
Drugs
Narrow Therapeutic Index Drugs
(NTIs) are defined as having less than a two-fold difference between the
minimum toxic concentrations and the minimum effective concentrations in the
blood.12 Some examples of NTIs are digoxin, lithium, phenytoin,
theophylline, and warfarin. These products do not have any specialized
conditions required by the FDA. Despite this and other misgivings with the
ANDA approval process, generic medications are generally accepted by
physicians and patients. There are some variables, though, that influence a
patient's acceptance of a generic substitution.

The Pharmacist's Role
The concern about generic
substitution among many patients stems from lack of information. They wonder
if generics are as strong as the brand. They worry that generics may cause
more adverse effects. Some have had a negative experience with a generic drug.
The pharmacist is perfectly
poised to educate patients on the use of generic drugs. When a patient drops
off a prescription, the pharmacist can take that opportunity to clear up any
confusion with regard to the use of generic products. Generic drugs are a
cost-containing alternative to higher priced brand name drugs. Informed
consumers are better equipped to make decisions regarding their prescriptions.
References
1. Generic Pharmacy Association
Statistics. www.gphaonline.org/Content/NavigationMenu/
AboutGenerics/Statistics/. Accessed April 4, 2008.
2. National Health Expenditures, average percent change, and percent distribution, by type of expenditure: United States, selected years 1960-2005. Health, United States. 379. www.cdc.gov/nchs/data/hus/hus07.pdf#summary. Accessed April 5, 2008.
3. Hoffman JM, Shah ND, Vermeulen LC, et al. Projecting future drug expenditures--2007. Am J Health Syst Pharm . 2007;64:298-314.
4. Savings from generic drugs purchased at retail pharmacies. Center for Drug Evaluation and Research, U.S. Food and Drug Administration. May 3, 2004. www.fda.gov/cder/consumerinfo/savingsfromgenericdrugs.htm. AccessedApril 19, 2008.
5. The new drug development process: steps from test tube to New Drug Application review. Center for Drug and Evaluation and Research, U.S. Food and Drug Administration. www.fda.gov/cder/handbook/develop.htm. Accessed April 18, 2008.
6. Welage LS, Kirking DM, Ascione FJ, Gaither CA. Understanding the scientific issues embedded in the generic approval process. J Am Pharm Assoc. 2001;41:856-867.
7. A bill to amend the patent laws of the United States. Public Law 98-417 (summary). Thomas, Law Library of Congress. http://thomas.loc.gov/cgi-bin/bdquery/z?d098:SN01538:@@@D&summ2=m&|TOM:/bss/d098query.html|. Accessed April 15, 2008.
8. Code of Federal Regulation-21 CFR 320.
9. Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther. 2003;23:2875-2890.
10. Approved Drug Products with Therapeutic Equivalence Evaluations. Rockville, MD: Center for Drug Evaluation and Research, U.S. Food and Drug Administration; 2008. www.fda.gov/cder/ob/. Accessed March 31, 2008.
11. Code of Federal Regulation-21 CFR 320.26.
12. Lung KR, Gorko MA, Llewelyn J, Wiggins N. Statistical method for the determination of equivalence in automated test procedures. J Auto Methods Manag Chem. 2003;25:123-127.
13. Statement of Janet Woodcock, MD,
Director, Center for Drug Evaluation and Research, before the subcommittee on
Oversight and Investigations Committee on Energy and Commerce.
http://google2.fda.gov/search?q=cache:vwaQUqwXxWgJ:www.fda.gov/ola/2008/heparin042908.html+GMP+drugs+china&access=p&output=
xml_no_dtd&ie=UTF8&lr=&client=FDA&proxystylesheet=FDA&oe=ISO-8859 1.
AccessedMay 9, 2008.
14. Agreement between the Department of Health and Human Services of the United States
of America and the State Food and Drug Administration of the People's Republic of
China on the Safety of Drugs and
Medical Devices. www.fda.gov/oia/agreements/
China_Drugsdevices.htm. Accessed May 9, 2008.
15. Ganther JM, Kreling DH. Consumer perceptions of risk and required cost savings for generic prescription drugs. J Am Pharm Assoc. 2000;40:378-383.
16. IMS Web site.
www.imshealth.com/ims/porta/pages/homeFlash/us/0,2764,6599,00.html. Accessed
May 9, 2008.






