US Pharm. 2015;40(11):HS3-HS9.
ABSTRACT: Traumatic brain injury (TBI) is a major cause of disability and death in the United States. With the devastating statistics of morbidity and mortality, swift medical attention is required to provide the best possible care in the hope of preventing long-term damaging effects. Various measures are employed in the hospital and ambulatory setting for individuals with TBI that address physical or cognitive sequelae of the traumatic event. Neuropsychiatric care in TBI patients includes the management of increased intracranial pressure, seizure occurrence, cognitive disturbances, and psychiatric disorders.
Traumatic brain injury (TBI) is a major cause of disability, death, and high healthcare costs in the United States that affects not only the injured persons but also their families and society.1-5 The effects of TBI include impaired thinking or memory, movement, sensation, or emotional functioning that, in some cases, can last a lifetime. TBI can be caused by an external force to the head that disrupts the normal function of the brain.1
Introduction
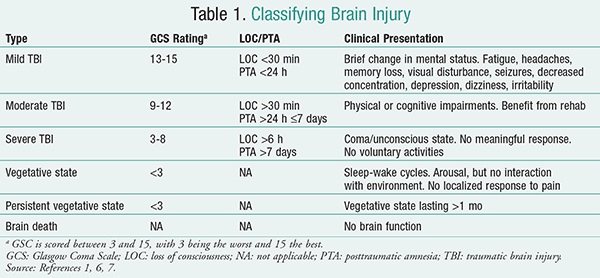
While all injuries to the head do not result in TBI, those that do cause an alteration of consciousness can be classified as either mild, moderate, or severe according to the Glasgow Coma Scale (GCS), loss of consciousness (LOC) time, and posttraumatic amnesia (PTA) time (TABLE 1).1,6,7
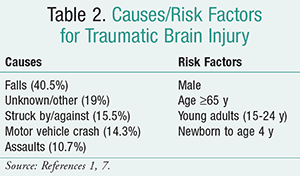
According to the CDC, an estimated 2.5 million emergency department (ED) visits, hospitalizations, or deaths were associated with TBI injuries in the U.S. in 2010. The leading causes of TBI from 2006-2010 were falls, motor vehicle crashes, and unintentional blunt trauma (TABLE 2).1,7
It is important to have valid estimates of brain injuries, characterize trends and demographics of those affected, and identify the major causes of TBI. This is useful for promoting prevention, predicting outcomes, focusing on wrap-around services needed, and developing targeted TBI-related interventions and best practices.3,4
The CDC currently uses sources that include the National Vital Statistics System Mortality Data, the National Hospital Discharge Survey (NHDS), and the National Hospital Ambulatory Medical Care Survey (NHAMCS). A recent article by Taylor et al called into question the use of the NHDS and the NHAMCS when estimating the national rates of TBI-related ED visits and hospitalizations given the rise in ED visits and the use of pooled data that can obscure year-to-year trends.4 The authors recognized the need to more carefully examine annual data in order to be able to quickly respond to emergent trends in the causes of TBI. A similar article by Leibson et al questioned the CDC’s data-systems approach with its potential for duplicate counting of TBI events and concluded that using the Rochester Epidemiology Project medical records-linkage resources (including highly sensitive and specific diagnostic coding) provided several advantages in estimating the incidence of TBI.3 There is an inherent need to be able to quickly recognize trends and produce programs that will promote prevention, thereby leading to reduced TBI-associated medical care costs along with improvement in currently available treatment of TBI, including those in the military veteran population.5,8
Acute Care Management
Emergency care of TBI continues to be a concern for public health in the U.S. With the devastating statistics of morbidity and mortality, swift medical attention is required to provide the best possible care in the hope of preventing long-term damaging effects. As previously stated, TBI can be classified as mild, moderate, or severe. This section will focus on moderate-to-severe TBI, which usually requires hospitalization.9
When brain injury occurs, the potential for brain damage and brain death ensues. Acute stabilization, preventing secondary injury, and restoring neuronal function are the goal for medical and pharmacologic interventions in an attempt to improve outcomes in this patient population.9
Diagnosis and assessment come through a history of external forces to the head or evident physical head trauma that causes decreased neurologic function. Laboratory tests like arterial blood gas, urine drug screen, blood alcohol concentration, and serum electrolytes are necessary to exclude other causes of neurologic dysfunction. Using the GCS and testing the reaction of the pupils to light should be a part of the initial examination. Some acute neurologic symptoms to monitor for include seizures, posttraumatic amnesia, dizziness, moderate-to-severe headache, limb weakness, and paresthesia.9
Treatment and prevention of acute neurologic sequelae are of utmost importance in the management of an acute TBI patient. Intracranial hypertension and seizure occurrence are common acute neuropsychiatric effects that need to be monitored for, addressed, and managed.
Although not the focus of this article, blood pressure control, venous thromboembolism prophylaxis, stress ulcer prophylaxis, glycemic control, possible mechanical ventilation, infection control, and nutrition support are all necessary management protocols to decrease morbidity and mortality in TBI, as with all other critical illnesses. The following will discuss management of intracranial hypertension and seizure occurrence.
Medical Management of Increased Intracranial Pressure
Intracranial pressure (ICP) is the pressure inside the skull and thus in the brain tissue and cerebrospinal fluid (CSF). After TBI, the brainstem may become compacted, causing compromised brain circulation, thus causing raised ICP. This is a critical condition and the most common cause of death in patients with severe TBI.10 Consequently, monitoring ICP is paramount in this patient population. The normal range for ICP is <10 to 15 mmHg in adults, 3 to 7 mmHg in children, and 1.5 to 6 mmHg in term infants. There are different thresholds for what is considered abnormal, thus requiring treatment. ICP thresholds of 15, 20, and 25 mmHg have been used in adults and 20 mmHg in pediatric patients.11,12
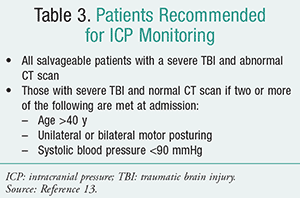
There are specific recommendations as to which patients should be monitored (TABLE 3).13 Benefits of ICP monitoring include early detection of intracranial mass lesion; guidance on therapy and avoidance of indiscriminate use of therapies to control ICP; drainage of CSF with reduction of ICP and improvement of cerebral perfusion pressure (CPP); and determination of prognosis. Clinicians should remember that when considering treatment for ICP, corticosteroids are not recommended in TBI patients for ICP reduction and also may be harmful.13
Sedation and Analgesia: Sedation and analgesia are common first-line therapy for intracranial hypertension. In severe TBI, patients are often very agitated, and sedation is usually required in spite of their ICP. It is usually unknown whether pain is the causative factor because of lack of communication due to brain injury. In addition, agitation in patients with TBI can increase ICP.9
Propofol is usually the drug of choice for sedation because of its rapid onset and short duration.9,13 Use of propofol must be balanced against the risk of adverse effects such as hypertriglyceridemia, infection, hypotension, overfeeding, and infusion-related syndrome. Midazolam is the preferred benzodiazepine because of its shorter half-life compared to lorazepam, easier titration, and status as the most studied drug in TBI.9 Since midazolam is not that effective in ICP control alone or in combination with propofol, it is used in patients who cannot receive propofol due to adverse effects. One study found that dexmedetomidine was safe and not associated with significant changes in intracranial hemodynamics.14
As for opioid therapy, morphine is widely used as a continuous infusion or intermittent bolus. Fentanyl and remifentanil are also used because of their shorter duration of action as a continuous infusion. Since intermittent bolus has been associated with likely increasing ICP, continuous infusion should be used whenever possible for the above mentioned opioids. These medications are recommended because they provide analgesia, mild sedation, and depression of airway reflexes, which is necessary for intubated and mechanically ventilated patients.9,13
Miscellaneous agents such as loop diuretics can be used as second-line therapy for fluid overload patients to decrease ICP. Neuromuscular blockers (NMBs) have also been used concomitantly for patients who are receiving maximum tolerated doses of propofol or who are refractory to all other agents for ICP control.9,11 Respiratory dyssynchrony, spontaneous respiratory efforts, muscle movements, muscle spasms, and oxygen consumption may all play a role in increasing ICP. Although NMBs may not have any direct effect on lowering of ICP, they may be beneficial for facilitating mechanical ventilation, minimizing muscle activity and spasms, and improving respiratory compliance.15
Hyperosmolar Therapy: Mannitol is very effective in lowering ICP in severe TBI. It works as an osmotic diuretic that creates an osmotic gradient, thereby increasing the serum osmolarity up to 310 to 320 mOSM/kg H2O in order to pull extracellular fluid from the brain. Mannitol is recommended for patients with signs of transtentorial herniation or progressive neurologic deterioration not attributable to extracranial causes. It is not recommended for prophylactic use or for patients with osmolarity >320 mOSM/kg H2O. In order to maintain euvolemia, adequate fluid replacement with isotonic saline solution should be used along with osmotic diuresis. Commonly used doses are 0.25 to 1 g/kg given IV over 15 to 20 minutes. Side effects to therapy are intravascular dehydration, hypotension, prerenal azotemia, and hyperkalemia. Sometimes mannitol may cause a rebound effect and lead to increased ICP. Its use is contraindicated in patients with TBI and renal failure because of the risk of pulmonary edema and heart failure.9,13
Hypertonic saline solution (HSS) 3% to 23.4% is an effective alternative to mannitol, and in some studies has been found to be superior. This alternative therapy causes osmotic dehydration and viscosity-related cerebral vasoconstriction. The beneficial effects of HSS in TBI include expansion of intravascular volume, extraction of water from the intracellular space, decrease in the ICP, controlled cerebral edema, and an increase in cardiac contractility. Hyperosmolarity effects such as renal failure and pulmonary edema are not associated with HSS. Central pontine demyelination, a neurologic condition associated with rapid increases in serum sodium levels, is unlikely to occur if the increase in serum sodium is <12 mEq/L per 24 hours. Therefore, HSS may be a good alternative or in some cases first-line hyperosmolar therapy.9,13
Temperature Modulation: Moderate hypothermia may be used in refractory, uncontrolled ICP. Moderate systemic hypothermia at 32°C to 34°C has shown evidence in reducing cerebral metabolism and cerebral blood volume, decreasing ICP, and increasing CPP. Although outcomes of temperature modulation in patients with TBI are controversial, temperature should be controlled and fever aggressively treated in this population.9,13
Barbiturate Coma: Barbiturates have been proven effective therapies for refractory or second-line intracranial hypertension. They reduce cerebral metabolism and cerebral blood flow and lower ICP. Pentobarbital is recommended for the induction of barbiturate coma. It is recommended in hemodynamically stable, severe TBI patients who are refractory to maximum medical and surgical ICP-lowering therapy. Common adverse effects of barbiturates include hypotension and immunosuppression. Prophylactic use of barbiturates is not recommended. Pentobarbital is given 10 mg/kg over 30 minutes, then 5 mg/kg/h for 3 hours, and then 1 mg/kg/h.9,12
Nonpharmacologic Therapy
There are also several nonpharmacologic therapies that may be beneficial in TBI. Elevating the head of the bed at a 30-degree angle in hemodynamically stable patients and CSF drainage in patients with ventriculostomy can be recommended. Both methods have been shown to decrease ICP.9 Surgical therapy with decompressive craniectomy or hemicraniectomy is an option for patients with acute severe TBI who are at risk for developing severe brain edema and intractable intracranial hypertension in cases where medical management fails. Decompressive therapy is a surgical procedure to control ICP in which part of the skull is removed to allow a swelling brain room to expand without being squeezed. Although decompressive therapy may be life-saving, evidence regarding its overall effects on outcomes is contradictory.13,16
Medical Management of Seizure Occurrence
According to the American Academy of Neurology, among all patients with head trauma who seek medical attention, about 2% develop posttraumatic seizures, although the number differs extensively depending primarily on injury severity. About 12% of patients with severe TBI develop posttraumatic seizures, and the rate may be greater than 50% for those with penetrating missile injuries.9
Posttraumatic seizures can be classified in two ways—as early or late onset. Early-onset seizures occur within 7 days of injury, and late-onset seizures occur 7 days after injury. Prophylactic therapy with antiepileptic drugs (AEDs) is not recommended for preventing late posttraumatic seizures. However, it is recommended for prophylactic therapy to prevent early posttraumatic seizure in TBI patients who are at high risk for seizures. High-risk patients are defined as having the following: GCS score <10, cortical contusion, depressed skull fracture, subdural hematoma, epidural hematoma, intracerebral hematoma, penetrating TBI, and seizures within 24 hours of injury. Phenytoin is the drug of choice for the prophylaxis of early posttraumatic seizures. A loading dose of 15 to 20 mg/kg administered IV over 30 minutes followed by 100 mg IV every 8 hours, titrated to recommended plasma level for 7 days. 9,13
Alternatively, levetiracetam has been studied and showed benefit, and is potentially as efficacious as phenytoin at doses of 500 to 1,000 mg twice daily for 7 days. A study involving levetiracetam in pediatric patients showed that it was effective and safe for early-onset seizure prophylaxis at a dosage of 10 mg/kg twice daily. Both valproate and phenobarbital have been studied and have demonstrated little benefit in posttraumatic seizures in high-risk TBI patients. Patients receiving AED prophylaxis should be monitored for potential side effects. Preventive therapy beyond the first 7 days of injury is not recommended.17-19
Outpatient Management
Various types of measures are employed in the ambulatory setting for individuals with TBI depending on the degree of impairment of the individual. These measures may address physical or cognitive sequelae of the traumatic event. Physical therapy and other rehabilitation measures are often employed to address the physical complications. Individuals can sometimes develop gait and balance disorders that can be resolved through physical therapy. Depending on the cause of the injury and extent of physical injury, situations where limb function, speech, and other bodily functions such as bladder and bowel function, swallowing, breathing, hormonal regulation, motor control, and blood pressure regulation are affected may require physical therapy, acute medical interventions, and other rehabilitation measures.20 Occupational therapy may also be beneficial in addressing home safety issues.21
There are some symptoms and complications that become apparent post injury that require varying levels of intervention, such as cognitive impairment and psychiatric disorders. The cognitive decline seen in posttraumatic injury may improve within 6 to 12 months, but may mimic some of the symptoms of various dementias.22 Full cognitive testing may rule out age-appropriate decline and allow for comprehensive analysis of other risk factors for dementia versus temporary cognitive decline. Attentional skills may be compromised and can easily be mistaken for dementia, so the age of the individual is an important factor to determine the route of evaluation and treatment course.23,24 The inability to relate to others, lack of proper coping skills in stressful situations, mood swings, apathy, difficulty planning, poor concentration, and irritability can also occur. Screening and monitoring for depression, anxiety, and sleep impairment are essential in TBI.
The prevalence of depression, anxiety, substance abuse, and other psychiatric disorders varies tremendously and is confounded by the lack of screening, treatment, and follow up.25 Fenton et al found that nearly 40% of patients admitted with varying stages of TBI had a psychiatric diagnosis 6 weeks after the injury.26 The presence of substance use or abuse prior to the injury and a history of prior brain injuries can negatively influence the prognosis. Posttraumatic stress disorder can also occur in TBI, so clinicians should screen accordingly and include as assessment of sleep quality.27 Pharmacotherapeutic treatment selection should be individualized and ideally be able to address comorbid disorders. Cognitive behavioral therapies are also highly effective as monotherapy or as adjuncts to pharmacotherapy.21,28
When medications are utilized for patients with TBI, it is imperative that lower doses be used and attention given to the impact of the drug on cognitive function, because individuals with TBI may be more sensitive to the effects of selected agents. When considering long-term pharmacotherapy for seizure management, attention should be paid to the risk of recurrent seizures and seizure type when deciding to initiate AEDs and choosing a drug.29,30 There are no FDA-approved medications for the treatment of neuropsychiatric symptoms of TBI, and treatment trials are lacking in this population. Therefore, it is important to treat only the target symptoms that the patient is experiencing. Before starting pharmacotherapy, always assess for spontaneous resolution of symptoms. Slow titration is very important in this population because of increased sensitivity to cognitive effects of medications. “Start low and go slow” is the best approach when it comes to titration. If there is not a beneficial response to medication, then it is not necessary to continue drug therapy. Always select agents that have low potential for adverse effects, such as sedative/cognitive, anticholinergic, extrapyramidal, seizure, and drug interactions. Some target symptoms that are managed include cognitive impairment, depression, agitation/aggression, psychosis, and sleep disturbances.31 TABLE 4 list drugs that are used off-label for neuropsychiatric sequelae in chronic TBI management.29-43

Follow-up is important, especially since many of those who have incurred mild TBI only develop difficulties, such as impaired problem-solving skills, irritability, poor ability in relating to others, and easy distractibility, after they resume daily activities and encounter stressful situations. Limiting the number of medications and using the minimal effective doses when possible are also necessary to minimize exposure to side effects that can impede cognitive recovery. Clinicians should develop a treatment plan that allows for maximal quality of life for the individual.
Conclusion and Pharmacist’s Role
As the medication experts, pharmacists are in the perfect position to effectively impact the acute and chronic care of patients with TBI. Pharmacists can improve medication outcomes by ensuring that guidelines for TBI are consistently being followed in patient care pathways (e.g., seizure prophylaxis, sedation/analgesia). Pharmacists can also ensure that any drugs that may worsen outcomes or increase the likelihood of adverse effects particular to this patient population are avoided (i.e., cognitive impairment). Adding a clinical pharmacist to a multidisciplinary team will allow for more therapeutic drug monitoring, medication reconciliation, drug interaction screening, and cost-saving initiatives, which can have a positive effect on this highly vulnerable patient population.
REFERENCES
1. CDC. Traumatic brain injury in the United States: fact sheet. www.cdc.gov/traumaticbraininjury/get_the_facts.html. Accessed July 21, 2015.
2. Lenrow D. What is traumatic brain injury (TBI)? TraumaticBrainInjury.com. www.traumaticbraininjury.com. Accessed July 21, 2015.
3. Leibson CL, Brown AW, Ransom JE, et al. Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology. 2011;22(6):836-844.
4. Taylor CA, Greenspan AI, Xu L, Kresnow MJ. Comparability of national estimates for traumatic brain injury-related medical encounters. J Head Trauma Rehabil. 2015;30(3): 150-159.
5. Leibson CL, Brown AW, Hall Long K, et al. Medical care costs associated with traumatic brain injury over the full spectrum of disease: a controlled population-based study. J Neurotrauma. 2012;29(11):2038-2049.
6. Ruff RM, Iverson GL, Barth JT, et al. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009;24(1):3-10.
7. CDC. Traumatic brain injury. www.cdc.gov/traumaticbraininjury. Accessed July 21, 2015.
8. Dismuke CE, Walker RJ, Egede LE. Utilization and cost of health services in individuals with traumatic brain injury. Glob J Health Sci. 2015;7(6):43213.
9. Wood GC, Boucher BA. Management of acute traumatic brain injury. PSAP-VIII: Neurology and Psychiatry. Washington, DC: American College of Clinical Pharmacy; 2013:139-156. www.accp.com/docs/bookstore/psap/p7b10sample03.pdf. Accessed September 11, 2015.
10. Romner B, Grande P. Intracranial pressure monitoring in traumatic brain injury. Nat Rev Neurol. 2013;9:185-186.
11. Dunn L. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;73(suppl I):i23–i27.
12. Kukreti V, Mohseni-Bod H, Drake J. Management of raised intracranial pressure in children with traumatic brain injury. J Pediatr Neurosci. 2014;9(3):207-215.
13. Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12.
14. Grille P, Biestro A, Fariña G, Miraballes R. Effects of dexmedetomidine on intracranial hemodynamics in severe head injured patients. Neurocirugia (Astur). 2005;16(5): 411-418.
15. Murray MJ, Cowen J, DeBlock H, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30(1):142-156.
16. Cooper DJ, Rosenfeld JV, Murray L, et al. Decom-pressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493-1502.
17. Shin Y, Benavides S, Wurster J, Patel N. Levetiracetam (Keppra) efficacy and safety in the prevention of early-onset seizures following traumatic brain injuries in pediatric patients. Mental Health Clin. 2015;5(4):144-148.
18. Korobey MJ. Chapter 8: Prevention of seizures following traumatic brain injury. In: Sadaka F, ed. Traumatic Brain Injury. Rijeka, Croatia: InTech; 2014: 168-186. www.intechopen.com/books/traumatic-brain-injury/prevention-of-seizures-following-traumatic-brain-injury. Accessed October 13, 2015.
19. Chang B, Lowenstein D. Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60:10-16.
20. Bland D, Zampieri C, Damiano D. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: a systematic review. Brain Inj. 2011;25(7-8):664-679.
21. Tomaszewski W, Manko G. An evaluation of the strategic approach to the rehabilitation of traumatic brain injury (TBI) patients. Med Sci Monit. 2011;17(9):CR510-CR516.
22. Neumann D, Lequerica A. Cognitive problems after traumatic brain injury. Arch Phys Med Rehabil. 2015;96(1): 179-180.
23. Catroppa C, Anderson V, Godfrey C, Rosenfeld JV. Attentional skills 10 years post-paediatric traumatic brain injury (TBI). Brain Inj. 2011;25(9):858-869.
24. Godbolt AK, Cancelliere C, Hincapié C, et al. Systematic review of the risk of dementia and chronic cognitive impairment after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95(3 suppl):S245-S256.
25. Deb S, Lyons I, Koutzoukis C. Rate of psychiatric illness 1 year after traumatic brain injury. Am J Psychiatry. 1999;156: 374-378.
26. Fenton G, McClelland R, Montgomery A, et al. The postconcussional syndrome: social antecedents and psycho-logical sequelae. Br J Psychiatry. 1993;162:493-497.
27. Bombardier C, Fann JR, Temkin N, et al. Posttraumatic stress disorder symptoms during the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2006;18(4):501-508.
28. Fann JR, Bombardier CH, Vannoy S, et al. Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. J Neurotrauma. 2015;32(1):45-57.
29. Dilley M, Cerian Avent C. Long-term neuropsychiatric disorders after traumatic brain injury. In: Uehara T, ed. Psychiatric Disorders: Worldwide Advances. Rijeka, Croatia: InTech; 2011.
30. Gallagher D. Post-traumatic epilepsy: an overview. Einstein Quart J Biol Med. 2002;19:5-9.
31. Fleminger S. Neuropsychiatric effects of traumatic brain injury. Psychiatric Times. March 9, 2010. www.psychiatrictimes.com/neuropsychiatry/neuropsychiatric-effects-traumatic-brain-injury. Accessed October 13, 2015.
32. Glenn MB, Wroblewski B, Parziale J, et al. Lithium carbonate for aggressive behavior or affective instability in ten brain-injured patients. Am J Phys Med Rehabil. 1989;68(5):221-226.
33. Hale MS, Donaldson JO. Lithium carbonate in the treatment of organic brain syndrome. J Ner Ment Dis. 1982;170(6):362-365.
34. Brooke MM, Patterson DR, Questad KA, et al. The treatment of agitation during initial hospitalization after traumatic brain injury. Arch Phys Med Rehabil. 1992;73:917-921.
35. Greendyke RM, Kanter DR, Schuster DB, et al. Propranolol treatment of assaultive patients with organic brain disease: a double-blind crossover, placebo-controlled study. J Ner Ment Dis. 1986;174(5):290–294.
36. Michals ML, Crismon ML, Roberts S, Childs A. Clozapine response and adverse effects in nine brain-injured patients. J Clin Psychopharmacol. 1993;13(3):198-203.
37. Kim E, Bijlani M. A pilot study of quetiapine treatment of aggression due to traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2006;18(4):547-549.
38. Noé E, Ferri J, Trénor C, Chirivella J. Efficacy of ziprasidone in controlling agitation during post-traumatic amnesia. Behav Neurol. 2007;18(1):7-11.
39. Scott LK, Green R, McCarthy PJ, Conrad SA. Agitation and/or aggression after traumatic brain injury in the pediatric population treated with ziprasidone. J Neurosurg Pediatr. 2009;3(6):484-487.
40. Du B, Shan A, Zhang Y, et al. Zolpidem arouses patients in vegetative state after brain injury: quantitative evaluation and indications. Am J Med Sci. 2014;347(3):178-182.
41. Azouvi P, Jokic C, Attal N, et al. Carbamazepine in agitation and aggressive behaviour following severe closed-head injury: results of an open trial. Brain Injury. 1999;13:797-804.
42. Warden DL, Gordon B, McAllister TW, et al; Neuro-behavioral Guidelines Working Group. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23:1468-1501.
43. Defense Centers of Excellence for Psychological and Traumatic Brain Injury clinical recommendation. Manage-ment of sleep disturbances following concussion/mild traumatic brain injury: guidance for primary care manage-ment in deployed and non-deployed settings. June 2014. https://dvbic.dcoe.mil/sites/default/files/2014_Sleep_CR_interactive_07.15.14.pdf. Accessed October 13, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






