US Pharm.
2007;32(3):45-50.
Acute renal failure (ARF) is defined as a
rapid loss of renal function due to damage to the kidneys. This results in
electrolyte and acid-base abnormalities and retention of nitrogenous waste
products, such as urea and creatinine.
Patients with ARF are often
asymptomatic and are diagnosed by observed elevations in blood urea nitrogen
(BUN) and serum creatinine (SCr) levels. Common symptoms of ARF include
anorexia, fatigue, mental status changes, nausea, vomiting, and pruritus.
Seizures can occur if BUN levels are extremely high, and shortness of breath
can result if volume overload is present.1 However, alterations in
urine volume may be the only symptom that patients notice.
Populations most at risk
include the elderly and those with underlying renal insufficiency. Conditions
that compromise renal blood flow or alter effective circulatory volume--such as
bilateral renal artery stenosis, cirrhosis, nephrotic syndrome, or congestive
heart failure--are considered risk factors for ARF.
Incidence and Reporting of
ARF
The incidence of
ARF, although relatively common, is difficult to define, and the incidence of
drug-induced renal failure (DIRF) is even more difficult to ascertain. Current
information suggests that ARF accounts for 1% of hospital admissions,
implicating occurrence in the outpatient setting, and occurs in 2% to 5% of
in-hospital patients. For hospitalized patients in the intensive care unit
(ICU), the occurrence rate is 1% to 25%, with a 15% occurrence for patients
undergoing cardiopulmonary bypass.1-4 Worldwide, the reported
incidence of ARF in critical illness is 1% to 25%, with 3.4% to 4.9% of
patients requiring renal replacement therapy (RRT).3 DIRF occurs in
18% to 27% of hospitalized patients with ARF, and 20% of hospital admissions
for ARF are reportedly caused by drugs, particularly nonsteroidal
anti-inflammatory drugs (NSAIDs).5
There are several explanations
for the lack of an accurate incidence of ARF in the population. First, there
is no universally accepted clinical definition for ARF. Historically, most
definitions have relied on an increase in the concentration of SCr (e.g., >0.5
mg/dL or 25%). A recent review of the epidemiology of ARF revealed that
approximately 35 definitions exist in the medical literature.3 With
such a variety of definitions, the range may widen for those definitions using
modest increases in SCr or narrow for those studies that use tighter criteria
to define ARF, such as the need for RRT.
The
Acute Dialysis Quality Initiative (ADQI), a group composed of nephrologists
and intensivists with expertise in renal disease, recently proposed the RIFLE
criteria for acute renal dysfunction. The RIFLE criteria evaluate severity and
outcome of ARF. The severity classes (Risk, Injury, and F
ailure) are based on the degree of change in urine output or SCr, and the
outcome classes (Loss and End-Stage Kidney Disease) are based on
the duration of kidney function loss.6
Several recent studies have
begun using the RIFLE criteria. One major limitation they have encountered is
that urine output cannot be accurately assessed without a urinary catheter,
and use of diuretics, which increase urine output, decreases the validity of
this measurement. Despite these limitations, the RIFLE criteria will provide a
more accurate determination of the incidence not only of ARF but of DIRF as
well.6
Determining the incidence of
DIRF is even more difficult, particularly in the community, because mild
changes in renal function often go unrecognized and unreported. Furthermore,
in-hospital occurrence rates are low, due to both underrecognition and
underreporting. Not all hospitals actively report adverse drug reactions, and
most data, if collected, remain unpublished.
Morbidity and Mortality
The mean age at
onset of ARF is approximately 67 years.7 In a recent prospective
study identifying 1,738 patients with an increase in plasma urea or renal
dysfunction that required dialysis, the median age was 67 years, and the mean
length of stay in the ICU was 10 days.8
The mortality rate for
patients with ARF is 23% to 80%, and this rate increases to 57% to 80% in
patients requiring RRT. Hospital mortality for critically ill patients with
ARF requiring RRT is approximately 60% to 70%.3 As with incidence
data for ARF, mortality data for ARF are inaccurate due to the lack of a
universally accepted definition for the condition and the disparate patient
populations that have been studied.
Most patients recover from ARF
by 90 days, with 60% to 70% of patients recovering without the need for RRT.
Patients with normal renal function prior to the first episode of ARF have a
lower likelihood of needing long-term RRT.9
Types of ARF
There are three
types of ARF--prerenal, intrinsic, and postrenal ARF--which are classified based
on underlying causes. Although there are multiple pathophysiologic causes for
each type of ARF, drugs are common precipitating factors for each category.
Prerenal ARF accounts for 40%
to 70% of cases and results from decreased perfusion to the kidney. It may be
caused by decreased intravascular volume due to blood loss, dehydration, or
disease states such as congestive heart failure, hypotension, and liver
failure, which result in decreased effective blood volume. Pre- and
postglomerular arteriolar resistance is responsible for maintaining renal
perfusion and glomerular filtration rate. Preglomerular (afferent)
vasodilation and post-glomerular (efferent) vasoconstriction are controlled by
prostaglandins and angiotensin II, respectively. Interruption of these
pathways by drugs such as NSAIDs and angiotensin-converting enzyme (ACE)
inhibitors results in renal hypoperfusion. Patients with underlying disease,
such as the elderly and those with hypotension and dehydration, are at
particular risk for DIRF.1,4,5 Considering the availability of
NSAIDs and the growing size of the aging population, the risk of developing
NSAID-induced renal failure is quite high.
Intrinsic ARF accounts for 10%
to 50% of ARFcases and results from damage to the kidney tissue. Various
inflammatory diseases, such as systemic lupus erythematosus, can result in
glomerulonephritis. Interstitial nephritis results from inflammation of the
renal interstitium and tubules and can be caused by infections,
immune-mediated diseases such as sarcoidosis and lymphomas, and drugs. Drugs
most often implicated in the development of interstitial nephritis include
certain antibiotics, antivirals, and immunosuppressants.4,10
Renal tubular injury usually
results from ischemia or drugs. The tubules have an inherently high-energy
demand due to active transport mechanisms and metabolic processes. This makes
the tubules particularly sensitive to decreases in oxygen. Drugs such as
amphotericin B upset the balance between oxygen demand and supply, which
results in tubular damage.5,10 Other drugs, such as
aminoglycosides, radiocontrast media, and heavy metals, become concentrated in
the kidney and cause a direct toxic effect, usually in a dose-dependent manner.
4,5,10
Postrenal ARF accounts for
only 10% of ARF cases and results from obstruction within the urinary tract
that prevents the outflow and elimination of urine.4 The
obstruction must involve both kidneys in order for ARF to develop.1
Patients at risk for postrenal ARF include those with malignancy, prostate
disease, and bladder-outlet obstruction. Drugs such as acyclovir and
methotrexate can cause crystal deposition in the tubules, which can occur when
a patient is dehydrated. Drugs with low solubility may form crystals, causing
obstruction of urine output and subsequent renal failure.
NSAIDs
Each year, up to 5% of people who
take NSAIDs will develop renal toxicity, resulting in hospital admissions and
an increase in health care spending.1
All NSAIDs inhibit
cyclooxygenase, the enzyme that is required to convert arachidonic acid into
prostaglandins. Prostaglandins are not only involved in the inflammatory
process but are present in the kidneys. They balance the effects of
vasoconstrictors (norepinephrine, angiotensin II, vasopressin) by causing
vasodilation of the afferent arteriole and, ultimately, allow adequate renal
blood supply and glomerular filtration pressure.
Unopposed vasoconstriction of
the afferent arteriole in a patient taking NSAIDs causes decreased blood flow
to the kidneys, which results in decreased glomerular filtration rate and
renal ischemia.
NSAIDs should be avoided or
used with caution in patients at high risk of renal failure. COX-2 inhibitors
are included in this warning due to similar effects on renal function.
Patients should continue taking aspirin for cardioprotection, because low
doses do not significantly affect prostaglandin levels in the kidneys.11
Patients taking high doses of
NSAIDs, individuals with underlying renal insufficiency, and the elderly are
at a greater risk of toxicity. Factors that cause decreased volume and/or
blood flow to the kidneys, such as congestive heart failure, cirrhosis,
dehydration, and overdiuresis, predispose patients to ARF.12 When
dispensing medications that can precipitate ARF, counsel patients on the risk
of using over-the-counter NSAIDs without consulting their pharmacist or
physician.
ACE Inhibitors and
Angiotensin II Receptor Blockers
ACE inhibitors and angiotensin II
receptor blockers are another frequent cause of ARF, especially in patients
with severe renal artery stenosis or chronic kidney disease and in those
hospitalized for congestive heart failure. Current guidelines recommend ACE
inhibitors for patients with chronic kidney disease and systolic heart failure
because of their proven benefits on morbidity and mortality;13
however, low doses should be used initially, and renal function should be
monitored frequently.
Glomerular pressure is
normally high enough to maintain adequate filtration without relying on
postglomerular resistance. In the setting of reduced blood flow, however,
glomerular filtration is dependent on resistance in the efferent arteriole
created by angiotensin II–mediated vasoconstriction. ACE inhibitors reduce the
outflow resistance from the glomerulus, resulting in decreased pressure and
glomerular filtration.
An increase in SCr of up to
30% is expected in the first two to five days of therapy with an ACE
inhibitor. This effect will stabilize after a few weeks of therapy and remain
until discontinuation of the drug.5 Treatment with an ACE inhibitor
should be stopped if SCr increases by more than 30% and reduced if
reinitiated. A mild decrease in renal function due to ACE inhibitors is
acceptable due to the benefits that result from long-term therapy.
Treatment should be started at
low dosages, especially in patients with underlying risk factors, and the dose
should be titrated gradually. It is important to avoid dehydration and
excessive use of diuretics and NSAIDs.
Aminoglycoside Antibiotics
Aminoglycosides are
used to treat infections with gram-negative bacteria. They cause
nephrotoxicity in up to 10% to 20% of patients when used for a full course of
therapy.14
The primary mechanism of
aminoglycoside-induced ARF is injury to the proximal tubule leading to
cellular necrosis. This occurs via binding of cationic charges on amino groups
to tubular epithelial cells. Tubular cell death occurs from generation of
oxygen-free radicals and subsequent alterations in cellular function.
Risk factors for
aminoglycoside-induced ARF include aminoglycoside dosing (i.e., large
cumulative dose, prolonged therapy, trough concentrations >2 mg/dL),
synergistic exposure to other nephrotoxins (especially concomitant
vancomycin), and underlying condition of the patient.
Typically, pharmacists monitor
aminoglycoside levels during inpatient treatment. Inherent pharmacodynamic and
pharmacokinetic properties of aminoglycosides have led to more frequent use of
once-daily dosing as opposed to traditional multiple daily-dosing regimens.
Aminoglycosides display concentration-dependent killing and significant
"postantibiotic" effect; therefore, giving a higher dose less frequently is at
least as effective and may decrease renal toxicity by allowing excretion of
aminoglycosides from the tubular cells prior to the next dose.12
SCr concentrations should be
monitored in patients receiving aminoglycosides. Renal toxicity is usually
seen in the first five to seven days of therapy but may occur earlier in
certain high-risk patients. If aminoglycosides are the treatment of choice,
ensuring adequate hydration and avoiding exposure to other nephrotoxic agents
is imperative and may prevent aminoglycoside-induced ARF.
Radiocontrast Dye
ARF is frequently
caused by administration of radiographic contrast dye (RCD), which is used for
diagnostic and treatment procedures. The incidence approaches nearly 50% in
patients with combined diabetes and pre-existing renal insufficiency.5
Other risk factors for RCD-induced ARF include volume depletion, high doses
of RCD, and using other drugs that cause nephrotoxicity.
Most patients experience a
transient rise in SCr within two to five days after receiving RCD, followed by
recovery to baseline over the next few days.15 High-risk patients
may experience more severe toxicity and require dialysis. Hospital course is
significantly affected due to comorbid conditions that worsen with the onset
of ARF.16
Nephrotoxicity appears to
result from a combination of direct tubular necrosis and renal ischemia.
Significant injury to the tubular cells and production of toxic-free radicals
occur after RCD and may be accompanied by renal vasoconstriction and ischemia.
Adequate hydration and
discontinuation of nephrotoxic drugs is an essential part of the prevention of
RCD-induced ARF. Many small trials have shown conflicting results regarding
the use of various fluids, bicarbonate, diuretics, and acetylcysteine for
prevention of RCD-induced ARF, and there are no clear recommendations based on
proven benefit.
Isotonic normal saline (1
mL/kg) may provide the most benefit and should be given at least six to 12
hours prior to RCD and continued six to 12 hours after the procedure.16
In addition, administration of sodium bicarbonate one hour prior to the
procedure, with continued treatment for at least six hours after RCD, may also
provide additional benefit.17 Though data are inconclusive at this
time, acetylcysteine (600 to 1,200 mg by mouth) given in two doses the day of
and after the procedure is reasonable based on low toxicity and cost.
Diuretics should be given only if the patient is fluid overloaded.15
Another ongoing debate
involves the choice of an RCD agent. The newer RCD agents have a lower
osmolality and have been associated with less renal toxicity in patients with
diabetes and renal insufficiency. These agents are significantly more
expensive than traditional RCD agents and do not completely eliminate
nephrotoxicity. Most experts recommend low osmolality agents only for
high-risk patients.18 Although this is a cost-effective strategy
for high-risk patients, it is not recommended for the entire population of
patients receiving RCD for a diagnostic procedure.
Other Drugs
Statin drugs, which
are used for hypercholesterolemia, are typically thought of in association
with elevated liver enzymes; however, statin drugs are associated with
rhabdomyolysis, which is known to cause ARF. Rhabdomyolysis leads to muscle
breakdown products in the circulation. ARF results from direct toxicity of
myoglobin and intravascular volume depletion, partly from muscle edema.12
Patients should be warned to go to the emergency department immediately if
they begin to experience a sudden increase in muscle pain and weakness,
especially if it is associated with an increase in temperature.
Despite approval of several
new antifungals, amphotericin B continues to be the drug of choice for
life-threatening systemic fungal infections. Dose-dependent acute tubular
necrosis occurs often and requires discontinuation of the drug.5
Many liposomal amphotericin B formulations that are associated with less
nephrotoxicity have been developed. These formulations are limited by their
cost but are currently recommended for patients with preexisting kidney
disease and those at a high risk of ARF.
A complete list of drugs and
the types of ARF that can result from toxicity can be found in Table 1.
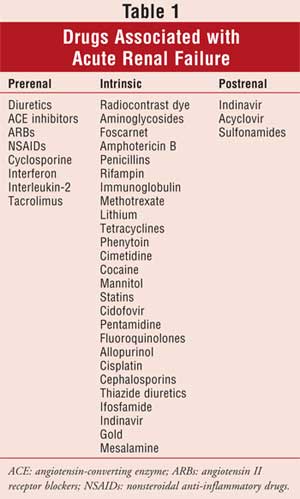
Summary
DIRF is a serious, and often
preventable, disease associated with significant morbidity and high health
care costs. Drugs are often the culprit, and they range from commonly used
over-the-counter analgesics to immunosuppressants and chemotherapeutic agents.
As more and more drugs are introduced into the market without a clearly
defined adverse drug reaction profile, recognition and reporting of potential
adverse drug reactions, including nephrotoxicity, are becoming more important
than ever. The FDA released a comprehensive statement early this year
committing to a new initiative focusing on drug safety,19 including
improving methods of surveillance to identify unforeseen drug toxicity.
The more pharmacists learn
about drugs that are used frequently among patients, the better prepared they
will be to help their patients make informed decisions. Prevention is the
treatment of choice for ARF, as well as DIRF. Identifying patients at high
risk is the first step. Patients should be counseled on concomitant
medications that might cause ARF and the risk of dehydration.
References
1. Mueller BA. Acute renal failure. In: Pharmacotherapy. 6th ed. New York, NY: McGraw-Hill; 2005;781-90.
2. Gill N, Nally JV Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005;128:2847-2863.
3. Uchino S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12:538-543.
4. Hilton R. Acute renal failure. BMJ. 2006;333:786-790.
5. Nolin TD, Himmelfarb J, Matzke GR. Drug-induced kidney disease. In: Pharmacotherapy. 6th ed. New York, NY: McGraw-Hill; 2005;871-87.
6. Hoste E, Kellum JA. Acute kidney injury: epidemiology and diagnostic criteria. Curr Opin Crit Care. 2006;12:531-537.
7. Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care. 2006;12:557-560.
8. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813-818.
9. Bagshaw SM. The long-term outcome after acute renal failure. Curr Opin Crit Care. 2006;12:561-566.
10. Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11:555-565.
11. Mene P, Pugliese F, Patrano C. The effects of nonsteroidal anti-inflammatory drugs on human hypertensive vascular disease. Semin Nephrol. 1995;15:244-252.
12. Guo X, Nzerue C. How to prevent, recognize, and treat drug-induced nephrotoxicity. Cleve Clin J Med. 2002;69:289-290,293-294,296-297.
13. Chobanian AV, Bakris GL, Black HR. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206-1252.
14. Swan SK. Aminoglycoside nephrotoxicity. Semin Nephrol. 1997;17:27-33.
15. Fry AC, Farrington K. Management of acute renal failure. Postgrad Med J. 2006;82:106-116.
16. Barrett BJ, Parfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386.
17. Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
18. Lin J, Bonventre JV. Prevention of radiocontrast nephropathy. Curr Opin Nephrol Hypertens. 2005;14:105-110.
19. The future of drug
safety--promoting and protecting the health of the public: FDA's response to
the Institute of Medicine's 2006 report. Released January 30, 2007. Available
at: www.fda.gov/oc/reports/iom013007.html.
To comment on this article, contact
editor@uspharmacist.com.






