US Pharm. 2010;35(10):HS-19-HS-25.
Note: All mentions of Mayo Clinic refer to the campus in Jacksonville, Florida.
Intravenous immunoglobulin (IVIG) products are commercially available preparations of immunoglobulins made from plasma pools obtained from more than 1,000 healthy donors per lot. IVIG consists of at least 90% intact immunoglobulin G (IgG). Four subclasses of IgG exist: IgG1, IgG2, IgG3, and IgG4. Of these, IgG1 is the major component in IVIG preparations. IgG1 is involved in tissue protection, complement activation, and virus inactivation. In addition, IgG1 makes bacterial cells susceptible to phagocytosis.1
Background
IVIG was originally used as antibody replacement therapy in patients with primary antibody deficiency to supplement the immune system. Use of IVIG has since expanded considerably to include the treatment and prevention of complications in various other conditions. IVIG is approved by the FDA for six indications: the treatment of primary immunodeficiencies; the prevention and control of bleeding in idiopathic thrombocytopenic purpura; the prevention of coronary artery aneurysms in Kawasaki disease; the prevention of infections and graft-versus-host disease in adult bone marrow transplant patients; the prevention of infections in chronic B-cell lymphocytic leukemia; and the prevention of infections and reduction of hospitalization time in pediatric patients with HIV infection. IVIG also is used off-label in the treatment of a number of conditions, including neurologic and neurocognitive disorders, solid-organ transplantation, infection-related diseases, dermatologic disorders, and recurrent spontaneous abortion.2-4
Availability
Although evidence supporting the use of IVIG products in many different disease states has been published, obtaining a sufficient supply of IVIG is challenging.5 IVIG has been in short supply and on allocation from manufacturers for many years. Several brands of IVIG products are marketed; they are generally considered equally effective, but they are not identical. These products are not rated as bioequivalent by the FDA, and differences in production method, virus elimination, and composition may demonstrate differences in safety and tolerability.6 Individual product differences allow prescribers to base their choice on individual patient needs in order to provide the patient with the safest, most tolerable, and most efficacious product.
Although IVIG remains in tight supply and is currently allocated based on prior usage, securing an allocation great enough to meet the anticipated demand for the upcoming year is typically achieved by Mayo Clinic (Jacksonville, Florida). However, the institution is not always able to obtain an allocation of preferred products.
When a supply of one of the allocated or preferred products is unavailable, the prescriber must decide whether therapy is required at that time or whether it can be delayed until the appropriate product is obtained. Frequently, prescribers who have to make this decision ask pharmacists to provide evidence supporting the use of alternative IVIG products for the patient’s indication and individual needs.
Mayo Clinic typically receives an allocation of Flebogamma (Grifols), which is used as the primary formulary product for patients requiring IVIG.7 The institution also receives smaller allocations of Gamunex (Talecris), Gammagard Liquid (Baxter), and Gammagard S/D (Baxter).8-10 Mayo Clinic restricts the use of Gamunex and Gammagard Liquid to patients with renal insufficiency or renal transplantation, and Gammagard S/D is restricted to immunoglobulin A–deficient patients.
Mayo Clinic is challenged to procure sufficient IVIG products for renal transplant patients. If a supply of formulary IVIG product restricted to patients with renal insufficiency or renal transplantation is unavailable, the pharmacy department is asked to obtain a sugar-free IVIG product. Receipt of this product is unlikely, however, since the institution has no prior history of its use.
The pharmacist is placed in a difficult position if the primary formulary product is unavailable, but the other restricted products are in stock. In such cases, the pharmacist needs to consider whether these products should be reserved for the intended patients or whether it is necessary to treat the patient in question at this time.
The purpose of this review is to differentiate IVIG products based on their use in patients with renal insufficiency or renal transplantation and to discuss the incidence of renal failure with IVIG products. This review is intended to serve as a resource to assist pharmacists in making the decision about whether to use an alternative IVIG product.
Stabilizers and Adverse Renal Events
The original formulations of IVIG, which was first approved in 1981, did not contain stabilizers. Undesirable side effects were seen with these early IVIG formulations, including fever; chills; fatigue; and chest, hip, joint, and back pain.11 These effects were thought to be due to the formation of immunoglobulin aggregates. Therefore, in an effort to reduce adverse effects, stabilizers were added to IVIG formulations to help decrease the aggregation of immune complexes. The added stabilizers are primarily sugars such as sucrose, maltose, glucose, and sorbitol. Glycine and albumin also may serve as stabilizers.
Unfortunately, these stabilizers may be implicated in the development of adverse renal events, including nonspecific acute renal failure, renal dysfunction, and osmotic nephrosis. The proposed mechanism of adverse renal effects is believed to be hyperosmolality caused by the stabilizers. Renal tubular injury occurs when sucrose, a large carbohydrate molecule, is taken up by tubular epithelial cells in a process called pinocytosis. This incorporation of sucrose into lysosomes causes swelling of the epithelial cells and cytoplasmic vacuolization of the tubules, especially in the proximal tubules. The swelling and vacuolization cause the tubular lumina to narrow, leading to injury and degeneration of the proximal tubular epithelium.12-14 Descriptions of the stabilizers and osmolality of IVIG products are given in TABLE 1.
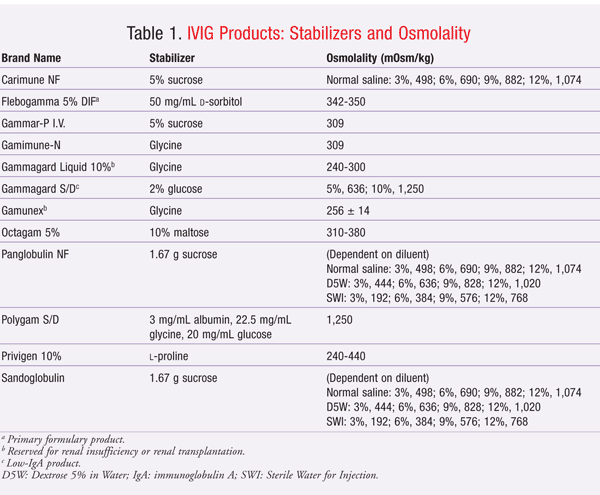
Sucrose is the IVIG stabilizer most commonly associated with adverse renal events, perhaps because sucrose has the highest osmotic activity of the stabilizers used in IVIG products. Moreover, sucrose is metabolized by an enzyme, called sucrase, that is found only in the intestine. All other stabilizers are metabolized by the liver. Since IVIG products are administered intravenously, sucrose cannot be metabolized in the intestine to glucose and fructose. Sucrose is eliminated unchanged in the urine, possibly resulting in osmotic nephrosis.13
Cautious use of IVIG is recommended in patients at increased risk for adverse renal events, including those with renal impairment, diabetes mellitus, age greater than 65 years, dehydration or hypovolemia, sepsis, paraproteinemia, or concomitant use of nephrotoxic drugs.15 Adverse renal events usually occur within 1 week (range, 1-10 days) of IVIG administration. Renal damage is usually reversible with discontinuation of therapy, and renal function typically returns to baseline within 2 weeks (range, 2-38 days). However, about 30% of patients who develop acute renal failure require short-term hemodialysis, and about 10% of cases are fatal.11,12
Incidence of Acute Renal Failure
Between June 1985 and November 1998, 120 cases of adverse renal events were documented worldwide, including 88 cases in the United States. Indications for IVIG use were reported in 97% of cases, including hematologic (46%), immunologic (23%), neurologic (20%), and infectious diseases (11%).16 Patient-specific information about renal-function status was not addressed. Of the 88 U.S. cases, 90% received sucrose-containing products (Panglobulin, Gammar-P I.V., or Sandoglobulin) and 8% received products containing maltose, glucose, albumin, or glycine (Gammagard S/D or Gamimune-N); the product was undetermined in the remaining 2%.16
Chapman et al reported two cases of acute renal failure following the administration of sucrose-stabilized IVIG (Panglobulin).13 Renal function returned to baseline after 11 days in one patient and 10 days in the other patient. In one case, acute renal failure did not recur after the patient received additional doses of a sorbitol-stabilized formulation (Venoglobulin-S). The other patient did not receive additional doses of IVIG. This report suggested that sucrose-stabilized IVIG products may be more likely than sorbitol-stabilized IVIG products to result in acute renal failure.
In the same paper, the investigators also provided a review of the published literature on renal adverse effects.13 Of 50 patient cases documented in the literature, the IVIG product was known in 38 cases. Of these, 37 patients received a sucrose-containing IVIG product and the remaining patient received a maltose-containing IVIG product.
Itkin and Trujillo reported acute renal failure in four patients who received IVIG formulations containing sucrose (Panglobulin).11 In each patient, renal function returned to baseline after therapy was discontinued. IVIG therapy with a nonsucrose-containing product (Polygam S/D) was continued in one patient, who experienced no further renal complications. The investigators also addressed formulary restrictions with IVIG.11 Panglobulin is the preferred IVIG product, regardless of renal failure risk, and Polygam S/D is considered only for patients who have developed acute renal failure and cannot tolerate Panglobulin therapy after further dilution of the drug and/or a decrease in infusion rate.
Renal Insufficiency and Renal Transplantation
Although all IVIG products carry a black box warning regarding the increased risk of renal dysfunction, they are not contraindicated in patients with renal insufficiency.7-10 IVIG is used in the treatment of certain complications of renal transplantation.4 IVIG has been studied to decrease anti–human leukocyte antigen (HLA) alloantibody titers before transplantation, to prevent rejection of organs after transplantation, as immunomodulatory medication after retransplantation, and as an alternative immunosuppressant option.17,18
Anti-HLA antibodies are present in up to 30% of patients waiting for a renal transplant.18,19 Moreover, 95% of patients with renal transplant rejection were positive for anti-HLA antibodies, versus 58% of patients with functioning transplants. The presence of these antibodies delays transplantation because a compatible organ must be found that does not contain HLA antibodies. It has been reported that identification of a compatible kidney may be delayed for 3 to 5 years.18,19 In addition to a delay in transplantation, patients with anti-HLA antibodies have an increased risk of rejection and a higher level of chronic graft dysfunction compared with antibody-naïve patients.18,19
The presence of HLA antibodies is determined by a test called crossmatching. A crossmatch (i.e., result indicating that the recipient will reject the potential donated organ) is determined by incubating serum from the transplant recipient with lymphocytes from the donor. The potential binding may be revealed through flow cytometry. A flow-positive crossmatch is not an absolute contraindication to transplantation, but it is associated with complications.
Although the mechanism of action is not completely understood, IVIG may benefit patients with anti-HLA antibodies in the following ways: neutralization of circulating antibodies; inhibition of the secretion of cytokines and other soluble mediators; inhibition of B- and T-cell proliferation, with downregulation of antibody synthesis; inhibition of endothelial-cell activation; inhibition of CD8 T-cell cytotoxicity; increased apoptosis of B cells; and inhibition of the maturation and function of dendritic cells.18
Practical Considerations
Practitioners should consider the following approaches to reduce the risk of acute renal failure when IVIG products are administered8-12:
• Reduce the administration rate to avoid large osmolar loads delivered over a short period of time.
• Provide adequate hydration prior to beginning the infusion.
• Use particular caution when administering IVIG to patients at high risk for renal failure.
• Do not exceed the recommended dose.
• Dilute the product to a concentration that will minimize the rate of delivery of stabilizer to the kidney.
• Assess the patient’s renal function after each treatment.
Conclusion
Products with lower osmolality or nonsucrose stabilizers may be preferred in patients with renal insufficiency; however, no direct comparison of the incidence of renal failure between IVIG products or specific stabilizers has been documented in the literature. Practitioners should collaborate to determine the best IVIG product for the patient based on product availability.
REFERENCES
1. MICROMEDEX online database [subscription required]. Greenwood Village, CO: Thomson Reuters (Healthcare), Inc. www.thomsonhc.com. Accessed September 14, 2010.
2. Leong H, Stachnik J, Bonk ME, Matuszewski KA. Unlabeled uses of intravenous immune globulin. Am J Health Syst Pharm. 2008;65:1815-1824.
3. Ratko TA, Burnett DA, Foulke GE, et al. Recommendations for off-label use of intravenously administered immunoglobulin preparations. University Hospital Consortium Expert Panel for Off-Label Use of Polyvalent Intravenously Administered Immunoglobulin Preparations. JAMA. 1995;273:1865-1870.
4. Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(suppl 4):S525-S553.
5. American Society of Health-System Pharmacists. ASHP drug product shortages management resource center. www.ashp.org/DrugShortages/Current/. Accessed September 14, 2010.
6. Shah S. Practical considerations for the use of IGIV therapy: introduction. Am J Health Syst Pharm. 2005;62(16 suppl 3):S3-S4.
7. Flebogamma (immune globulin intravenous [human]) product information. Los Angeles, CA: Grifols Biologicals, Inc; January 2010.
8. Gamunex (immune globulin intravenous [human]) product information. Research Triangle Park, NC: Talecris Biotherapeutics Inc; October 2008.
9. Gammagard Liquid (immune globulin, intravenous [human]) product information. Westlake Village, CA: Baxter Healthcare Corp; October 2009.
10. Gammagard S/D (immune globulin intravenous [human]) product information. Westlake Village, CA: Baxter Healthcare Corp; December 2009.
11. Itkin YM, Trujillo TC. Intravenous immunoglobulin-associated acute renal failure: case series and literature review. Pharmacotherapy. 2005;25:886-892.
12. Shah S, Vervan M. Use of i.v. immune globulin and occurrence of associated acute renal failure and thrombosis. Am J Health Syst Pharm. 2005;62:720-725.
13. Chapman SA, Gilkerson KL, Davin TD, Pritzker MR. Acute renal failure and intravenous immune globulin: occurs with sucrose-stabilized, but not with d-sorbitol–stabilized, formulation. Ann Pharmacother. 2004;38:2059-2067.
14. Cayco AV, Perazella MA, Hayslett JP. Renal insufficiency after intravenous immune globulin therapy: a report of two cases and an analysis of the literature. J Am Soc Nephrol. 1997;8:1788-1794.
15. FDA. Dear doctor letter—important drug warning: immune globulin intravenous (human). November 13, 1998. www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ucm105914.htm. Accessed September 14, 2010.
16. Renal insufficiency and failure associated with immune globulin Intravenous therapy—United States, 1985-1998. MMWR. 1999;48:518-521.
17. Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion. 2006;46:741-753.
18. Glotz D, Antoine C, Julia P, et al. Intravenous immunoglobulins and transplantation for patients with anti-HLA antibodies. Transpl Int. 2004;17:1-8.
19. Lee P, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009;88:568-574.
To comment on this article, contact rdavidson@uspharmacist.com.






