US Pharm.
2007;32(10):40-41.
Iron deficiency, the most common
nutritional deficiency worldwide, varies in prevalence according to age, race,
and sex (Table 1).1,2 According to data from the National
Health and Nutrition Examination Survey (NHANES 1999–2000), iron deficiency is
most prevalent among young children ages 1 to 2 years and adolescents and
adult females ages 12 to 49 years.1 Iron deficiency may have
potentially harmful effects in children, such as developmental delays and
behavioral disturbances. In pregnant women, it increases the risk of preterm
labor and low-birth-weight babies.

Anemia is a condition
characterized by abnormally low levels of healthy red blood cells or
hemoglobin (the component of red blood cells that delivers oxygen to tissues
throughout the body).3 A reduction in the amount of circulating
hemoglobin decreases the oxygen-carrying capacity of the blood. However, few
clinical symptoms may be present before hemoglobin levels fall below 7 to 8
g/dL.4 Patients with anemia may experience symptoms such as
tiredness, shortness of breath on exertion, cold intolerance, pallor of the
skin, ringing in the ears, dizziness, and confusion. To prevent iron
deficiency, at risk adults should consume iron-rich foods, and infants should
be administered iron-fortified formula.
Screening Methods
Anemia is not a
diagnosis but a symptom of underlying pathological conditions that requires
further investigation to determine its etiology. It can not be appropriately
diagnosed by clinical presentation alone. In clinical practice, anemia is most
often recognized by measurement of the red blood cell count, hemoglobin
concentration, and hematocrit. Until recently, laboratory tests for hemoglobin
were available exclusively through physician visits.
The BIOSAFE Anemia Meter
The BIOSAFEAnemia
Meter is the first FDA-approved, hand-held device that can be conveniently
used at home to test hemoglobin levels (Figure 1). Low levels of
hemoglobin may indicate anemia.5 Thus, the Anemia Meter may be used
as an additional screening method. It is not recommended for use in patients
younger than 18.
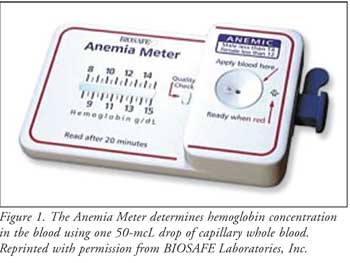
The device requires one 50-mcL
drop of capillary whole blood, of which 15 mcL is used to determine hemoglobin
concentration.6 After blood is collected, end point results are
rapidly seen within 20 minutes. Results are stored for one month, affording
patients an opportunity to talk with a health care professional about their
results. It is important to note that this device is not intended to replace
physician visits. Confirmation by professional laboratory methods is required
for results that are less than 8 g/dL or greater than 15 g/dL. Additionally,
if the test results do not indicate the possibility of anemia, patients should
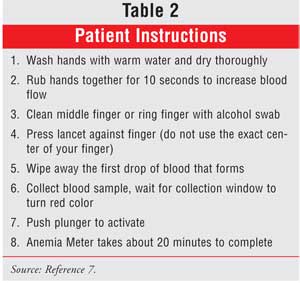
follow-up with their doctor. Patient instructions are provided in Table 2
.
Clinical Efficacy
In a double-blind clinical trial,
224 volunteer participants self-collected capillary whole blood samples and
tested for hemoglobin using the Anemia Meter. Paired venous samples were
obtained and analyzed for hemoglobin using the Abbott Cell Dyn Hematology
Analyzer.6 The range of hemoglobin values used was 5.9 to 18.2
g/dL. For females, the analytical specificity and accuracy was 98.9% and
97.1%, respectively. For males, the analytical specificity and accuracy was
shown to be 96.3% and 95.9%, respectively. It was concluded that the Anemia
Meter is more than 95% accurate and 96% specific when used correctly by
patients.
A side-by-side testing between
volunteers and medical professionals concluded that by following the
instructions in the package insert, untrained users could obtain hemoglobin
levels as accurate as those taken by professionals.6 Reliability
was tested by measuring the same blood sample multiple times under the same
conditions on five different devices. The aforementioned blood sample was then
tested on four different days. Variations were shown to be less than 6% for
both precision studies.
Conclusion
Although the prevalence of iron
deficiency anemia appears to be less common in the United States than in
developing countries, it remains above the Healthy People 2010
objectives of 5%, 1%, and 7% for toddlers, preschool children, and females
ages 12 to 49 years, respectively.2 For this reason, continued
monitoring of the iron status of the U.S. population is necessary in
vulnerable patients. The Anemia Meter offers an accurate, simple and
convenient method of monitoring hemoglobin levels, a key indicator of anemia.
For more information, visit the BIOSAFE Web site at www.ebiosafe.com or call
(800) 200-TEST (8378).
References
1. Centers for Disease Control and Prevention. Iron deficiency--United States, 1999-2000. MMWR Morb Mortal Wkly Rep. 2002;51:?897-899.
2. Killip S, Bennett J, Chambers M. Iron Deficiency Anemia. Am Fam Physician. 2007;75:671-678.
3. Ineck B, Mason B, Thompson E. Anemias, In: Dipiro J, Talbert R, Yee G et al., eds. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New York, NY: McGraw-Hill; 2005:1805-1831.
4. Glader B. Anemias, In: Behrman R, Kliegman R, Jenson H. eds. Nelson Textbook of Pediatrics. 17th ed. Pennsylvania:Saunders; 2004:1604-1605.
5. Weiss G, Goodnough L. Anemia of Chronic Disease. N Engl J Med. 2005;352:1011-1023.
6. BIOSAFE Medical Technologies, Inc. (BMT)
Technical Bulletin. Available at: www.ebiosafe.com. Accessed August 8, 2007.
7. BIOSAFE Medical Technologies, Inc.
(BMT) Anemia Meter Test Instructions. Available at: www.ebiosafe.com. Accessed
August 8, 2007.
To comment on this article, contact
editor@uspharmacist.com.






