US Pharm.
2008;33(5):HS14-HS19.
Candida
is a genus of opportunistic pathogens that affect high-risk patients who are
immunosuppressed or critically ill. Candidemia affects approximately one in
5,000 patients, with about 60,000 cases of candidemia occurring annually in
the United States.1,2 Candida is the fourth-leading cause of
nosocomial bloodstream infection today.3 The literature indicates
that 8% to 15% of all nosocomial bloodstream infections are caused by
Candida species.4 Candidemia carries with it a crude mortality
of 40%, which is one of the highest of all nosocomial bloodstream infections.
5 Depending on the population being evaluated, mortality rates range
from 57% in surgical patients to 78% in oncology patients.5
The literature suggests that
25% to 50% of nosocomial candidemia occurs in the critical care setting or
intensive care unit.3 Symptoms can range from low-grade temperature
to systemic inflammatory response with multiorgan failure. The lack of
reliable diagnostic tests also makes early detection difficult. Candidemia is
associated with higher rates of mortality and morbidity, as well as increased
costs, underscoring the need for safer and more effective therapies.2,4
Patients should be evaluated for their risk of developing invasive
candidiasis or candidemia, with empiric therapy initiated as quickly as
possible (Table 1).6

Candida
species are the most common cause of fungal infections, with Candida
albicans being the most frequently isolated species.1,3,7 There
has been a shift, with non-albicans Candida being responsible for
invasive candidiasis in hospitalized patients caused by Candida tropicalis
, Candida glabrata, Candida parapsilosis, and Candida krusei
. These non-albicans species are becoming an increasing problem,
especially in patients with acute life-threatening candidal infections.
1,3,7
Patients developing
candidemia, on average, add 10 days to the length of their hospital stay, at a
cost of approximately $39,000 per patient.8 The estimated cost (in
1997 U.S. dollars) to treat an episode of candidemia was $34,123 per Medicare
patient and $44,536 per private insurance patient. The major cost associated
with candidemia is that of increased length of stay.8
A retrospective study by
Zaoutis et al estimated the incidence of candidemia in hospitalized children
and adults in the U.S. in 2000.9 In adult patients, candidemia was
associated with a mean 10.1-day increase in length of stay and a mean increase
of hospital charges of $39,331 (ranging from $33,604 to $45,602).
Fluconazole
The Infectious
Diseases Society of America (IDSA) recommends fluconazole (Diflucan) as an
option for initial treatment of presumed Candida infections.7
Other therapies include amphotericin B (Ambisome, Amphocin, Fungizone),
caspofungin (Cancidas), flucytosine (Ancobon), itraconazole (Sporanox), and
voriconazole (VFEND). The decision of which agent to choose should depend on
the local epidemiology of the specific institution, the patient's clinical
status, prior exposure to azole compounds, and the potential for drug-induced
toxicities that compromise the outcome.7 This review will focus on
fluconazole.
Fluconazole is a member of the
azole family, which inhibits the synthesis of ergosterol of the fungal cell
membrane.10 In vitro, the azoles are fungistatic against Candida
species. Fluconazole is available as IV and oral formulations. Oral
fluconazole is very well absorbed, and the bioavailability is greater than 90%.
11 Studies have shown that fluconazole 400 mg daily and amphotericin B
deoxycholate showed similar efficacy as empirical treatment for candidemia in
neutropenic and nonneutropenic patients, with fluconazole being associated
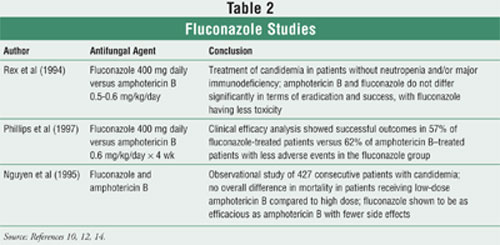
with fewer side effects (Table 2).10,12-14
Most of the adverse effects
associated with fluconazole are mild to moderate in severity. The most common
treatment-related adverse events reported were headache, nausea, vomiting,
diarrhea, and abdominal pain. Fluconazole has been associated with rare cases
of serious hepatic toxicity, which has usually been reversible on
discontinuation of therapy.11
In patients with impaired
renal function, the dose of fluconazole may need to be reduced because the
drug is primarily cleared by renal excretion. It may be useful to monitor
renal function, especially in elderly patients.11
Major Studies
Zerr et al
conducted a meta-analysis comparing amphotericin B deoxycholate and
fluconazole.15 The meta-analysis attempted to summarize the
efficacies of fluconazole and amphotericin B deoxycholate in treating invasive
candidiasis. They found no statistically significant differences with respect
to mortality. The authors found a statistical trend favoring amphotericin B
deoxycholate with respect to clinical failure. Unfortunately, the individual
trials included in the analysis and the meta-analysis were not powered to
detect a difference of clinical failure.
The National Committee for
Clinical Laboratory Standards developed standardized reproducible and
clinically relevant susceptibility testing methods for fungi.16
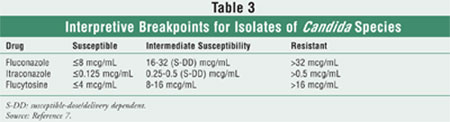
Data-driven interpretive breakpoints using this method are available for
testing the susceptibility of Candida species to fluconazole,
itraconazole, and flucytosine (Table 3).17-20 The
interpretive breakpoints place a strong emphasis on interpretation in the
context of the delivered dose of the azole antifungal agent. The category
susceptibility-dose/delivery dependent (S-DD) indicates that
maximization of the dose and bioavailability are critical to a successful
outcome (minimum inhibitory concentration [MIC] 16-32 mcg/mL). For
fluconazole, data for both humans and animals suggest that S-DD isolates may
be treated successfully with a dose of 12-mg/kg daily (800 mg daily for a
70-kg patient).19,21
Animal studies have
demonstrated that the ratio of the area under the concentration-time curve
(AUC) to the MIC best predicts the response to fluconazole therapy.22,23
This observation has potential clinical relevance since the AUC of
fluconazole in healthy adults is virtually identical to the daily dose in
milligrams.23 Rex et al demonstrated that a fluconazole dose to
48-hour MIC ratio of less than 25 correlated with an increased likelihood of
therapeutic failure.17,18,24
A study by Clancy et al tested
32 Candida isolates from blood cultures of patients with candidemia for
in vitro susceptibility to fluconazole and determined if the MIC and/or the
daily dose of fluconazole to MIC ratio correlated with the response to
therapy. Eighty-seven percent of patients were treated with 200 mg or less of
fluconazole daily, which contributed to a therapeutic failure in 53% of the
cases (17/32).25 Clancy's study revealed that a dose to MIC ratio
greater than 50 was associated with a success rate of 74% (14/19) compared to
8% (1/13) for a dose to MIC ratio less than or equal to 50. The therapeutic
success rate among patients infected with susceptible isolates (MIC ?8
mcg/mL) was 67% (14/21), susceptible-dose dependent (MIC 16-32 mcg/mL) was 20%
(1/5), and resistant (MIC ?64 mcg/mL) was 0% (0/6).25
In another study, 20
solid-tumor transplant patients with candidemia were treated with 600 or 800
mg of fluconazole daily. Three of the patients had isolates with an MIC of 32
mcg/mL (one patient) and an MIC of 64 mcg/mL (two patients). The only patient
in the study who failed therapy was receiving 600 mg daily and had an isolate
with an MIC of 64 mcg/mL.26
Munoz et al showed that three
patients with candidemia and infected with sensitive dose-dependent isolates
with an MIC of 16 mcg/mL were treated with 200 to 400 mg of fluconazole and
failed to respond.27 This would suggest that to achieve a dose to
MIC ratio of greater than 50, the minimum dose of fluconazole should be 6
mg/kg (400 mg for a 70-kg patient) and preferably 12 mg/kg (800 mg for a 70-kg
patient) for isolates with S-DD (MIC 16-32 mcg/mL).
Mortality has been shown to
increase in patients with candidemia who receive inappropriate fluconazole
therapy. Garey et al performed a retrospective cohort study of patients with
candidemia who were prescribed fluconazole at the onset of candidemia or later.
28 Total cost was lowest for patients receiving an adequate dose of
fluconazole on the day of fungal cultures. After controlling for covariates,
each one-day delay in fluconazole therapy was associated with increased total
hospital costs, and an adequate fluconazole dose was associated with decreased
total hospital costs.28 A delay or an inadequate dose in patients
with candidemia was associated with increased hospital costs. Inappropriate
antimicrobial therapy has been shown to be an important independent risk
factor for mortality among hospitalized patients with serious infection,
including bloodstream infections.29,30 Changing empiric
antimicrobial therapy to an appropriate regimen after identification of a
microorganism and its susceptibility does not improve clinical outcomes.
31,32 A study by Morrell et al demonstrated that the administration of
appropriate antifungal therapy more than 12 hours after the first positive
culture is drawn is associated with hospital mortality.33
These studies suggest that
administration of appropriate empiric therapy (drug and dose) to patients with
serious infections including candidemia should be initiated as soon as
possible.
Conclusion
The IDSA recommends
fluconazole as an option for initial therapy of presumed candidemia. It also
recommends a dose of at least 6-mg/kg daily (12-mg/kg daily for S-DD
isolates). Clancy's data suggest that both fluconazole MIC and dose to MIC
ratio correlate with the therapeutic response to fluconazole in patients with
candidemia. Since candidemia is associated with increased cost and mortality,
the dose of fluconazole in patients at risk for developing candidemia should
be 6- to 12-mg/kg daily (400-800 mg daily). Patients unable to receive
high-dose fluconazole (e.g., patients with renal insufficiency) should receive
an alternate antifungal agent.
REFERENCES
1. Rangel-Frausto
MS, Wiblin T, Blumberg HM, et al. National Epidemiology of Mycoses Survey
(NEMIS): variations in rates of blood stream infections due to Candida
species in seven surgical intensive-care units, and six neonatal-intensive
care units. Clin Infect Dis.1999;29:253-258.
2. McNeil MM, Nash SL,
Hajjeh RA, et al. Trends in mortality due to invasive mycotic disease in the
United States, 1980-1997. Clin Infect Dis. 2001;33:641-647.
3. Syndman D. Shifting
patterns in the epidemiology of nosocomial Candida infections. Chest.
2003;123:500S-503S.
4. Pittel D, Tarara D,
Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess
length of stay, extra costs, and attributable mortality. JAMA.
1994;271:1598-1601.
5. Wey SB, Mori M,
Pfaller A, et al. Hospital acquired candidemia. The attributable mortality and
excess length of stay. Arch Intern Med. 1988;148:2642-2645.
6. Blumberg HM, Jarvis
WR, Soucie JM, et al; National Epidemiology of Mycosis Survey (NEMIS) Study
Group. Risk factors for candidal bloodstream infections in surgical intensive
care unit patients: the NEMIS prospective multicenter study. Clin Infect
Dis. 2001;33:177-186.
7. Pappas PG, Rex JH,
Sobel JD, et al; Infectious Diseases Society of America. Guidelines for the
treatment of candidiasis. Clin Infect Dis. 2004;38:161-189.
8. Rentz AM, Halpern
MT. The impact of candidemia on length of hospital stay, outcome, and overall
cost of illness. Clin Infect Dis. 1998;27:781-788.
9. Zaoutis TE, Argon J,
Chu J, et al. The epidemiology and attributable outcomes of candidemia in
adults and children hospitalized in the United States: a propensity analysis.
Clin Infect Dis. 2005;41:1232-1239.
10. Rex JH, Bennett JE,
Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B
for treatment of candidemia in patients without neutropenia. N Engl J Med
. 1994;331:1325-1330.
11. Diflucan
(fluconazole) package insert. New York, NY: Pfizer Inc; August 2004.
12. Phillips P, Shafran
S, Garber G, et al. Multicenter randomized trial of fluconazole versus
amphotericin B for treatment of candidemia in non-neutropenic patients. Eur
J Clin Microbiol Infect Dis. 1997;16:337-345.
13. Anaaisse EJ, Rex
JH, Uzun O, et al. Predictors of adverse outcome in cancer patients with
candidemia. Am J Med. 1998;104:238-245.
14. Nguyen MH, Peacock
JE Jr, Tanner DC, et al. Therapeutic approaches in patients with candidemia:
evaluation in a multicenter, prospective, observational study. Arc Intern
Med. 1995;155:2429-2435.
15. Zerr DM, Garrison
MM, Marr KA, et al. A meta-analysis of fluconazole versus amphotericin B for
treatment of invasive Candida infections. JCOM. 2002;9:191-196.
16. The National
Committee for Clinical Laboratory Standards (NCCLS). Reference Method for
Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard.
NCCLS document M27-A2. Wayne, PA: NCCLS; 2002.
17. Rex JH, Pfaller MA.
Has antifungal susceptibility testing come of age? Clin Infect Dis.
2002;35:982-989.
18. Rex JH, Pfaller MA,
Galgiani JN, et al. Development of interpretive breakpoints for antifungal
susceptibility testing conceptual framework and analysis of in vitro-in vivo
correlation data for fluconazole, itraconazole, and Candida infections.
Clin Infect Dis. 1997;24:235-247.
19. Clancy CJ, Kauffman
CA, Morris A, et al. Correlation of fluconazole MIC and response to therapy
for patients with candidemia due to C. albicans and non-C. albicans
spp: results of a multicenter prospective study of candidemia. Presented at:
36th Annual Meeting of the Infectious Diseases Society of America; November
12-15, 1998; Denver, CO. Abstract 98.
20. Revanker SG,
Kirkpatrick WR, McAtee RK, et al. A randomized trial of continuous or
intermittent therapy with fluconazole for oropharyngeal candidiasis in
HIV-infected patients: clinical outcomes and development of fluconazole
resistance. Am J Med. 1998;105:7-11.
21. Andes D, van Ogtrop
M. Characterization and quantitation of the pharmacodynamics of fluconazole in
a neutropenic murine disseminated candidiasis infection model. Antimicrob
Agents Chemother. 1999;43:2116-2120.
22. Louie A, Drusano
GL, Banerjee P, et al. Pharmacodynamics of fluconazole in a murine model of
systemic candidiasis. Antimicrob Agents Chemother.
1998;42:1105-1109.
23. Louie A, Liu Q,
Drusano GL, et al. Pharmacokinetic studies of fluconazole in rabbits
characterizing doses which achieve peak levels in serum and area under the
concentration time curve values which mimic those of high dose fluconazole in
humans. Antimicrob Agents Chemother. 1998;42:1512-1514.
24. Rex JH, Pfaller MA,
Walsh TJ, et al. Antifungal susceptibility testing: practical aspects and
current challenges. Clin Microbiol Rev. 2001;14:643-658.
25. Clancy CJ, Yu VL,
Morris AJ, et al. Fluconazole MIC and fluconazole dose/MIC ratio correlates
with therapeutic response among patients with candidemia. Antimicrob Agents
Chemother. 2005;49:3171-3177.
26. Torres HA,
Kontoyiannis DP, Rolston KV. High-dose fluconazole therapy for cancer patients
with solid tumors and candidemia: an observational, noncomparative
retrospective study. Support Care Cancer. 2004;12:511-516.
27. Munoz P,
Fernandez-Turegano CP, Alcala L, et al. Frequency and clinical significance of
blood stream infections caused by C. albicans strains with reduced
susceptibility to fluconazole. Diagn Microbiol Infect Dis.
2002;44:163-167.
28. Garey KW, Turpin
RS, Bearden DT, et al. Economic analysis of inadequate fluconazole therapy in
non-neutropenic patients with candidemia: a multi-institutional study.
Internat J Antimicrob Agents. 2007;29:557-562.
29. Ibrahim EH, Sherman
G, Ward S, et al. The influence of inadequate antimicrobial treatment of
bloodstream infections on patient outcomes in the ICU setting. Chest.
2000;118:146-155.
30. Kollef MH, Sherman
G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk
factor for hospital mortality among critically ill patients. Chest.
1999;115:462-474.
31. Kollef MH, Ward S.
The influence of mini-BAL cultures on patient outcomes: implications for the
antibiotic management of ventilator associated pneumonia. Chest.
1998;113:412-420.
32. Rello J, Gallego D,
Mariscal D, et al. The value of routine microbial investigation in ventilator
associated pneumonia. Am J Respir Crit Care Med. 1997;156:196-200.
33. Morrell M, Fraser
VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream
infection until positive blood culture results are obtained: a potential risk
factor for hospital mortality. Antimicrob Agents Chemother.
2005;49:3640-3645.
To comment on this article,
contact rdavidson@jobson.com.





