Seizures are brief, paroxysmal, and abnormal neuronal discharges in the brain. About 10% of the population will experience a seizure at some point during their lifetime. In most of these individuals, seizures will not recur after the underlying cause has been corrected. Epilepsy, which is the recurrence of unprovoked seizures, occurs in about 1% of the population. There are approximately 200,000 new cases each year.1,2
Most newly diagnosed epileptic patients will become seizure free on their initial antiepileptic drug (AED) therapy; however, about 30% continue to experience seizures despite trials with multiple AEDs and are diagnosed as having refractory epilepsy.3-6 Studies show that patients who fail their first AED are less likely to become seizure free with each subsequent trial of another AED. This indicates that some patients have difficult-to-control epilepsy from the start. Conversely, some patients only show evidence of refractoriness to AEDs after several years of therapy.5-7
Some early indications that a patient might be resistant to treatment include young age at onset, multiple seizures prior to receiving treatment, seizures occurring in clusters, seizure type, abnormal neurologic exam, and developmental delay.5,6
In general, these patients are referred to epilepsy centers to receive comprehensive epilepsy care and are treated with combinations of different AEDs. However, there is evidence that even combination therapy with the most efficacious AEDs results in 50% improvement in only 32% to 37% of these patients.6 Nondrug options available to these individuals include epilepsy surgery, vagus nerve stimulation (VNS), and ketogenic diet.
Causes of Treatment Failure
Misdiagnosis of seizure type is a common cause of poor outcomes and should be considered before a patient is labeled with refractory epilepsy. Seizures are divided into partial (onset limited to one cerebral hemisphere) or generalized (onset involving both cerebral hemispheres). Partial seizures are further divided into simple partial (consciousness preserved), complex partial, or secondarily generalized (consciousness impaired). Generalized seizures are classified as convulsive or nonconvulsive types and are divided into several major categories (TABLE 1). Partial seizures are the most common type of seizure and the most difficult to control. Treatment failure can be the result of inappropriate selection of an AED for the specific type of seizure. For example, carbamazepine is a potent and efficacious AED and can be used to treat many types of seizures; however, it is well known to aggravate myoclonic and absence seizures.2-4,6

Poor compliance or noncompliance is also a common cause for treatment failure.4,6 In this case, selecting an AED with a better side-effect profile and a more simplified daily regimen may improve compliance and outcomes. To prevent seizure recurrence, patients should be educated early on about certain precipitating factors such as sleep deprivation, excessive alcohol consumption, intercurrent illnesses, and use of medications, such as tricyclic antidepressants, selective serotonin reuptake inhibitors, or bupropion, which may lower seizure threshold.
Antiepileptic Drug Therapy
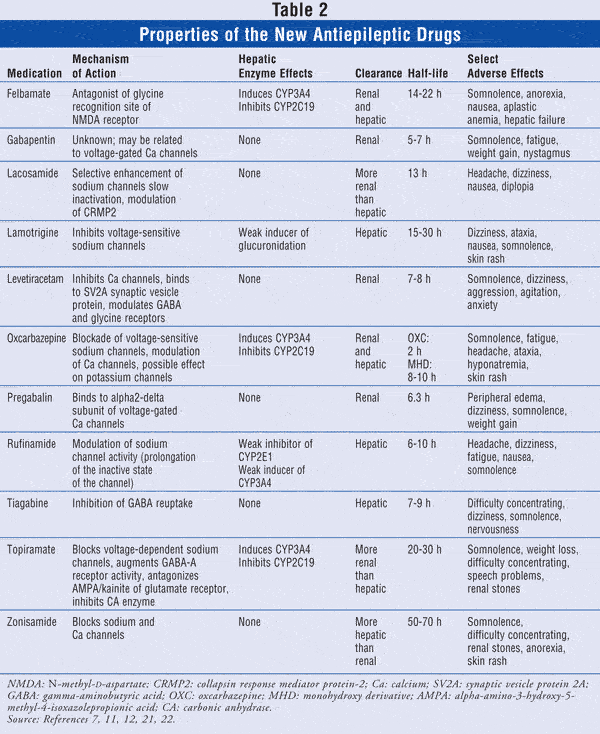
Over the past 15 years, 11 new AEDs have been approved in the United States (TABLE 2), expanding the number of combinations available to manage difficult-to-treat epilepsies. The new generation of AEDs offers similar efficacy compared to the older drugs (i.e., carbamazepine, phenytoin, phenobarbital, primidone, valproic acid), but with better side-effect profiles and fewer drug–drug interactions. The appropriate combination and optimal use of these drugs require knowledge of their mechanisms of action, pharmacokinetics, and pharmacodynamics.7
The mechanisms of some of the new AEDs are not completely understood. However, AEDs are generally grouped based on three mechanisms of action: 1) modulation of voltage-gated calcium, sodium, or potassium ion channels; 2) enhancement of gamma-aminobutyric acid (GABA)–mediated inhibition; and 3) reduction of the excitatory glutaminergic neurotransmission by blocking either the alpha-amino-3-hydroxy-5-
Evidence shows improved efficacy or increased tolerability when AEDs with different mechanisms of action and side-effect profiles are combined. Data suggest that combining certain medications may result in therapeutic synergy and benefit patients with generalized tonic-clonic and partial seizures. These medications include a sodium-channel blocker with a GABA-ergic inhibitor, two drugs with GABA-ergic properties, and an NMDA antagonist with an AMPA antagonist. Combinations meeting these criteria include phenytoin/valproic acid, valproic acid/carbamazepine, valproic acid/lamotrigine, carbamazepine/topiramate, phenobarbital/topiramate, felbamate/topiramate, oxcarbazepine/topiramate, and lamotrigine/topiramate.3,9,10
New Developments: A number of drugs in different stages of development may offer an alternative in patients with refractory epilepsy. Some of these are synthesized to enhance and improve the activity and adverse effect profiles of existing AEDs, including brivaracetam (levetiracetam analog), valrocemide (valproate analog), licarbazepine (oxcarbazepine analog), and fluorofelbamate (felbamate analog). Others have unique structures and may exert their activity through a novel mode of action.7
Two such drugs, lacosamide and rufinamide, were recently approved by the FDA.11,12 Lacosamide, a functionalized amino acid with a dual mode of action, is indicated as adjunctive therapy in treatment of partial seizures in patients aged 17 years and older. It is available as both a tablet and for injection; the IV formulation is approved for temporary use when the oral administration is not feasible. Rufinamide is a triazole derivative and is indicated for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in adults and in children as young as 4 years. It is available in tablet form for oral administration. In clinical trials, patients in rufinamide and lacosamide groups showed significant improvement in seizure frequency compared with placebo. Both medications were relatively well tolerated and the most common adverse effects reported included dizziness, nausea, and headache.11,12
Pharmacoresistance
The development of refractoriness to AED therapy is not very clear; however, two hypotheses have been proposed. Based on the target hypothesis, changes and alterations in AED targets such as ion channels or neurotransmitter receptors may lead to poor drug response.13-16 The transporter hypothesis postulates that overexpression of multidrug transporters such as P-glycoprotein in the blood–brain barrier may impair drug penetration into the brain and cause multidrug resistance in epilepsy. These changes may be intrinsic (i.e., genetically determined) or acquired (i.e., secondary to recurrent seizures). These hypotheses have been investigated in animal models as well as the brain tissue of refractory epileptic patients; however, support for their clinical relevance is lacking.13-16
Epilepsy Surgery
Brain surgery is an alternative treatment for patients with refractory epilepsy. The best candidates for surgery are individuals with focal epilepsy whose seizures arise from one area that is not critical for brain function. Because of significant advancements made in imaging and surgical techniques, it is possible to identify and remove the origin of seizures (seizure focus) more accurately. For carefully selected patients, epilepsy surgery can bring seizure freedom or a significant reduction in seizure frequency.3,17
There are two main types of surgery in epilepsy. The most common type is resective surgery, which consists of removing the seizure-generating focus. Resection of the mesial (medial) temporal lobe sclerosis (scar) accounts for about 75% of adult surgical procedures. In this procedure, the scar tissue or the seizure focus is surgically removed. On average, 48% to 84% of these patients have reported seizure freedom after this temporal lobectomy. The second and less common type is a palliative procedure to prevent the progression of seizure by interrupting the nerve pathways. In corpus callosotomy, the corpus callosum connecting the two hemispheres of the brain is disconnected, preventing progression and generalization of the seizure. This is an effective procedure—there have been reports of 80% reduction in generalized tonic-clonic seizures and 50% reduction in complex partial, myoclonic, and absence seizures.3,17,18
Other Therapies
Vagus Nerve Stimulation: Other therapies available to patients with refractory epilepsy include VNS and ketogenic diet. VNS is a nonpharmacologic tool that can be considered as an adjunctive therapy for patients with refractory partial seizures who are not candidates for epilepsy surgery or are unwilling to undergo such procedures. The pulse generator of this device is implanted subcutaneously in the left infraclavicular region and is connected to the left vagus nerve. Studies show 50% improvement in seizure frequency in 37% of patients in their first year of therapy; however, seizure freedom is rare.19
Ketogenic Diet: Ketogenic diet is a high-fat, low-carbohydrate diet that has shown efficacy in treatment of refractory epilepsy. Although the mechanism of its antiepileptic activity is not completely understood, new evidence suggest that the production of ketone bodies may diminish hyperexcitability of neurons and improve seizure control.20 However, its potential complications, such as metabolic acidosis, hypoglycemia, dehydration, and hyperlipidemia, should be discussed before considering this diet.
Conclusion
Despite the availability of new drugs and the expansion of our options for treating epilepsy, at least 30% of newly diagnosed individuals remain refractory to current treatment. Improved understanding of the causes of refractoriness and development of novel agents hold the promise of therapeutic outcomes in the near future.
Patients with refractory epilepsy are usually treated with multiple AEDs, and because of the chronic nature of this disease, comprehensive care for these individuals should include their sociopsychological needs as well as the management of the chronic side effects of these medications. Pharmacists with a good knowledge of the pharmacokinetic and pharmacodynamic properties of AEDs can play a significant role in the management of individual drug therapy and subsequently improve patient outcomes. This can be achieved by adjusting medication doses based on serum levels and patients’ response and tolerance, anticipating drug–drug interactions and adverse drug reactions, and improving compliance by educating patients about their medications.
REFERENCES
1. Epilepsy Foundation of America. Epilepsy and seizures statistics. www.epilepsyfoundation.org/
2. Shneker BF, Fountain NB. Epilepsy. Dis Mon. 2003;49:426-478.
3. Schuele Su, Luders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514-524.
4. Devinsky O. Patients with refractory seizures. N Engl J Med. 1999;340:1545-1570.
5. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;324:314-319.
6. French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48(suppl 1):53-57.
7. Leeman BA, Cole AJ. Advancements in the treatment of epilepsy. Annu Rev Med. 2008;59:503-523.
8. Vajda FJ. Pharmacotherapy of epilepsy: new armamentarium, new issues. J Clin Neurosci. 2007;14:813-823.
9. Deckers CL, Czuczwar SJ, Hekster YA, et al. Selection of antiepileptic drug polytherapy based on mechanisms of action: the evidence reviewed. Epilepsia. 2000;41:1364-1374.
10. Brodie MJ. Modern approaches to epilepsy management. N Am Pharmacother. 2004:2;195-197.
11. Doty P, Rudd GD, Stoehr T, Thomas D. Lacosamide. Neurotherapeutics. 2007;4:145-148.
12. Arroyo S. Rufinamide. Neurotherapeutics. 2007;4:155-162.
13. Sanchez Alvarez JC, Serrano Castro PJ, Serratosa Fernandez JM. Clinical implications of mechanisms of resistance to antiepileptic drugs. Neurologist. 2007;13(suppl 1):S38-S46.
14. Kwan P, Brodie M. Potential role of drug transporters in the pathogenesis of medically intractable epilepsy. Epilepsia. 2005;46:224-235.
15. Loscher W. Drug transporters in the epileptic brain. Epilepsia. 2007;48(suppl 1):S8-S13.
16. Rogawski MA. What clinical observations on the epidemiology of antiepileptic drug intractability tell us about the mechanisms of pharmacoresistance. Department of Neurology. UC Davis School of Medicine. July 17, 2008. http://repositories.cdlib.org/
usdavisneurology/rogawski5. Accessed October 1, 2008.
17. Duncan JS. Epilepsy surgery. Clin Med. 2007;7:137-142.
18. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol.
2008;7:525-537.
19. Ramani R. Vagus nerve stimulation therapy for seizures. Neurosurg Anesthesiol. 2008;20:29-35.
20. Kim DY, Rho JM. The ketogenic diet and epilepsy. Curr Opin Nutr Metab Care. 2008;11:113-120.
21. Fischer JH. Drug Interactions with Antiepileptic Agents 2006. New York, NY: McMahon Publishing Group; 2006.
22. Johannessen SI, Tomson T. Pharmacokinetics variability of newer antiepileptic drugs: when is monitoring needed? Clin Pharmacokinet. 2006;45:1061-1075.
To comment on this article, contact rdavidson@jobson.com.





