US Pharm. 2011;37(1):HS6-HS8.
Drug-induced lupus (DIL) is one of the most widely described drug-induced rheumatologic syndromes.1,2 It has been estimated that up to 10% of systemic lupus erythematosus (SLE) cases are drug induced, which approximates to 15,000 to 30,000 cases per year.3,4 Sulfadiazine was the first drug to be implicated in the causation of lupus in 1945.5 A definite association between hydralazine and lupus was first described in 1953.6 In order to meet criteria for a drug-induced syndrome, the patient should have exposure to the offending agent, clinical and laboratory evidence for a rheumatologic syndrome without prior evidence of such a disorder, and rapid improvement of symptoms and serologic findings upon discontinuation of the drug.2
Clinical Description
DIL is a syndrome that resembles a milder version of idiopathic SLE and presents after the exposure to an offending medication. Patients typically experience arthralgia, arthritis, myalgia, and serositis.1,2,7 TABLE 1 provides a description of the clinical characteristics of DIL.2,7,8 Arthralgia is present in approximately 90% of these patients and is sometimes the only clinical symptom present.1 Myalgia is also present in approximately half of all cases of DIL. Other systemic features that can occur are fever, hepatomegaly, skin eruptions such as a rash or erythema, photosensitivity, splenomegaly, pericarditis, and the presence of antinuclear antibodies (ANAs).1,2 With drugs such as procainamide, hydralazine, sulfasalazine, isoniazid, and carbamazepine, the pericardial involvement has been reported to reach a cardiac tamponade.1 The malar rash, alopecia, and photosensitivity that are commonly observed in SLE can also be present in DIL but at a lower incidence.1,2 The frequency of the clinical symptoms can vary depending upon the agent. In most cases, DIL induces autoantibodies at a higher frequency than in SLE, and this is an important immunologic characteristic of the disease.8,9
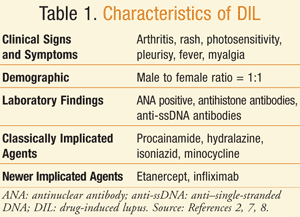
Approximately 90% of DIL cases have positive ANAs present in a homogenous pattern.1 Antihistone antibodies are positive in greater than 75% of patients in DIL and in approximately 20% of SLE patients. The antihistone antibodies in DIL primarily form against a complex of the histone dimer H2A-H2B, which is in contrast to idiopathic SLE in which complexes are formed against the H1 and H2B subunits.10 In DIL, there are also higher frequencies of anti–single-stranded DNA (anti-ssDNA) antibodies, as compared to SLE. Anti–double-stranded (anti-dsDNA) antibodies are rare with DIL due to hydralazine, procainamide, and isoniazid.1,11 On the contrary, anti-dsDNA antibodies are seen with DIL due to interferon-alfa and anti–tumor necrosis factor alpha (anti-TNF-alpha) agents.11 Renal and neurologic involvement is also rare.1,3
Drug-induced subacute cutaneous lupus erythematosus (SCLE) can be triggered by drugs such as terbinafine, hydrochlorothiazide, etanercept, and calcium channel blockers.1,12,13 Patients present with erythema, photosensitivity, and skin lesions. ANAs and histone antibodies are also present.1,8
Drug-induced chronic cutaneous lupus erythematosus (CCLE) is rare and usually related to fluorouracil agents, although infliximab and etanercept have been implicated in causing this syndrome as well.7,13 The duration of treatment with the offending agent reported before the development of drug-induced CCLE was 8 months.1 Skin lesions and positive ANAs were seen in the majority of cases.1,7
Criteria for DIL
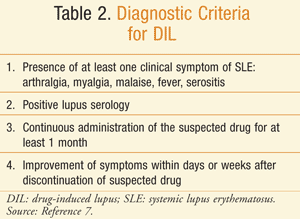
The diagnosis of DIL can be difficult, as there are no set criteria. TABLE 2 provides a summary of the guidelines for diagnosing DIL.7 The diagnosis is usually based upon a clinical picture that is consistent with lupus, the history of the disease and disease onset, and a demonstrated relationship with the offending agent.1 Proposed criteria are the presence of at least one clinical symptom of SLE and positive ANAs or other lupus serology (especially positive immunoglobulin [Ig]G antihistone antibodies); the administration of a suspected drug over an appropriate period of time (3 weeks to 2 years) before the development of any sign or symptom used as one of the criteria for the SLE diagnosis; and improvement of clinical signs after discontinuation of the suspected drug within 1 year and recurrence of symptoms upon rechallenge. The patient can still exhibit positive laboratory findings for a longer period of time.1,14
Drugs Capable of Inducing Lupus
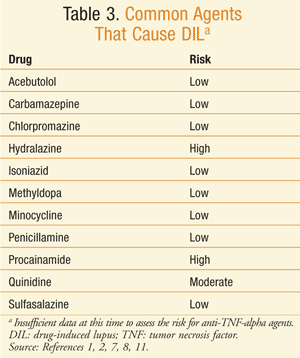
Drugs have been identified as a definite, probable, or possible causation of DIL. At least 80 drugs that can cause DIL have been identified.2 Some of the medications that have been identified as capable of definitely causing DIL are procainamide, hydralazine, minocycline, penicillamine, isoniazid, quinidine, and anti-TNF-alpha agents (TABLE 3).3,15-20 The drugs with the highest risk of DIL are procainamide with a 20% incidence and hydralazine with a 5% to 8% incidence of DIL for 1 year of therapy at the usual dose.8
Procainamide: Procainamide has the highest involvement with DIL. Positive ANAs were seen with almost all patients who were given the drug for 2 years or more, and symptoms occurred in almost one-third of the patients who took procainamide for more than 1 year.19
Hydralazine: The incidence of DIL with hydralazine is lower than that with procainamide. Risk factors for DIL with hydralazine have been identified and include the drug dose (>200 mg/day and/or a cumulative dose of >100 g reported in a mean time of 24 months), slow hepatic acetylation, female gender, and HLA-DR4 genotype.19-22 Approximately one-third of patients will test positive for the antihistone antibody.
Minocycline: Minocycline was the first tetracycline to be linked with DIL, and currently at least 78 cases of minocycline-induced DIL have been reported.23 Tetracyclines have been reported to exacerbate existing SLE after a short period of administration, as well as latent SLE and photosensitivity.24 One study described 15 cases of DIL that were confirmed after rechallenge with minocycline.25 DIL from minocycline can take from 3 months to 6 years to develop; therefore, the majority of the cases reported are from treatment of acne and rheumatoid arthritis.1,25
Typical symptoms of minocycline-induced lupus include arthralgia, arthritis, fever, morning stiffness, and myalgia.23,26-27 Raynaud’s disease and mouth ulcers have also been reported.23
There are contrasts in the presentation of the clinical signs and symptoms of DIL due to minocycline. There is a higher female to male ratio (5:1).23 Positive ANAs are seen but antihistone antibodies are rarely positive.25 Anti-dsDNA antibodies are also seen. Perinuclear antineutrophil cytoplasmic antibodies were positive in 67% of the cases assayed.25
Newer Agents: Anti-TNF-alpha agents are used in the treatment of diseases such as rheumatoid arthritis and psoriasis. Case reports for DIL have been reported with etanercept and infliximab.28-30 The clinical trial data for infliximab reported higher numbers of ANAs and anti-dsDNA antibodies than those in the control group.31 Five cases of DIL due to infliximab were reported in the clinical trials among 2,292 patients treated.30 Data from the clinical trial of etancercept reported that 11% of etanercept patients were positive for ANAs and 15% were positive for anti-dsDNA (compared to 5% and 4%, respectively, in the placebo group). Fever, malaise, arthritis, and rash were reported. Renal and neurologic involvement was rarely reported.1
Treatment
The mainstay of treatment for DIL is to withdraw the offending agent; symptom improvement can be seen within days to weeks after discontinuation of the drug.8 Supportive therapy may also be prescribed to help patients with the symptoms of DIL.32 Arthralgia and arthritis can be treated with nonsteroidal anti-inflammatory drugs (NSAIDs) or low-dose corticosteroids.14 For the treatment of the cutaneous manifestations of DIL, which include rash or skin lesions from drug-induced SCLE, low-dose systemic corticosteroids or topical corticosteroids in combination with hydroxychloroquine may be used.11
In drug-induced CCLE, topical corticosteroids in combination with hydroxychloroquine have been used.13 For severe symptoms, such as pleuropericarditis, severe arthritis, or DIL that affects the neurologic and renal systems, high-dose systemic corticosteroids can be given for 2 to 10 weeks.14 The doses prescribed are similar to those used for the treatment of arthralgia, arthritis, or rash, as are the side effects experienced.
NSAIDs: For example, ibuprofen (400-800 mg/dose 3-4 times/day, maximum 3.2 g/day) or naproxen (500-1,000 mg/day in 2 divided doses) may be used. The possible side effects that can occur include but are not limited to dizziness, drowsiness, headache, pruritus, edema, and fluid retention.33,34
Topical Corticosteroids: Hydrocortisone, betamethasone, and fluticasone are some examples of topical corticosteroids that may be used for skin rash or skin lesions. A thin layer of the substance should be applied to the affected area two to three times daily. Common side effects are dryness, itching, skin atrophy, and erythema.35
Systemic Corticosteroids: Prednisone and methylprednisolone are often used to treat symptoms of DIL. Low-dose treatment is typically 10 mg or less per day, and high-dose treatment is typically 1 mg/kg/day. The side effects commonly seen are headache, mood swings, fluid and electrolyte imbalance, insomnia, peptic ulcers, and urticaria.36,37
Hydroxychloroquine: The dose commonly used is 400 mg once or twice daily. Side effects typically seen are nausea, vomiting, diarrhea, alopecia, corneal changes and deposits, and pigmentation changes.38
Conclusion
Many drugs currently available are capable of inducing autoantibodies, which can lead to a drug-induced disease such as DIL. Thus far, at least 80 medications have been identified that can cause DIL. Specific criteria for the diagnosis of DIL have not been defined, but recommendations have been made to aid in its diagnosis. There are three forms of DIL currently recognized: systemic DIL, drug-induced SCLE, and drug-induced CCLE.
New drugs are increasingly entering the market with a mechanism of action that modifies the immunologic response in the patient. For pharmacists, an awareness of the potential adverse effects of these medications can help with the identification of this drug-induced syndrome in patients. Patients should also be counseled to be aware of the signs and symptoms of DIL and encouraged to report any concerns to their physician.
REFERENCES
1. Antonov D, Kazandjieva J, Etugov D, et al. Drug-induced lupus erythematosus. Clin Dermatol. 2004;22:157-166.
2. Brogan BL, Olsen NJ. Drug-induced rheumatic syndromes. Curr Opin Rheumatol. 2003;15:76-80.
3. Hess E. Drug-related lupus. N Engl J Med. 1988;318:1460-1462.
4. Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci. 2007;1108:166-182.
5. Hoffman BJ. Sensitivity to sulfadiazine resembling acute disseminated lupus erythematosus. Arch Dermatol Syph. 1945;51:190-192.
6. Morrow JD, Schroeder HA, Perry HM Jr. Studies on the control of hypertension by hyphex. II. Toxic reactions and side effects. Circulation. 1953;8:829-839.
7. Katz U, Zandman-Goddard G. Drug-induced lupus: an update. Autoimmun Rev. 2010;10:46-50.
8. Rubin RL. Etiology and mechanisms of drug-induced lupus. Curr Opin Rheumatol. 1999;11:357-363.
9. Rubin RL. Drug-induced lupus. Toxicology. 2005;209:135-147.
10. Yung RL, Johnson KJ, Richardson BC. New concepts in the pathogenesis of drug-induced lupus. Lab Invest. 1995;73:746-759.
11. De Bandt M. Lessons for lupus from tumour necrosis factor blockade. Lupus. 2006;15:762-767.
12. Callen JP, Hughes AP, Kulp-Shorten C. Subacute cutaneous lupus erythematosus induced or exacerbated by terbinafine: a report of 5 cases. Arch Dermatol. 2001;137:1196-1198.
13. Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatologic aspects. Lupus. 2009;18:935-940.
14. di Fazano CS, Bertin P. The pharmacological management of drug-induced rheumatic disorders. Expert Opin Pharmacother. 2001;2:1623-1631.
15. Fritzler MJ. Drugs recently associated with lupus syndromes. Lupus. 1994;3:455-459.
16. Shakoor N, Michalska M, Harris CA, Block JA. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet. 2002;359:579-580.
17. De Rycke L, Kruithof E, Van Damme N, et al. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondyloarthropathy. Arthritis Rheum. 2003;48:1015-1023.
18. Atzeni F, Turiel M, Capsoni F, et al. Autoimmunity and anti-TNF-alpha agents. Ann N Y Acad Sci. 2005;1051:559-569.
19. Yung R, Richardson B. Drug-induced rheumatic syndromes. Bull Rheum Dis. 2002;51:1-6.
20. Cameron HA, Ramsay LE. The lupus syndrome induced by hydralazine: a common complication with low dose treatment. Br Med J. 1984;289:410-412.
21. Batchelor JR, Welsh KI, Tinoco RM, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet. 1980;1:1107-1109.
22. Speirs C, Fielder AH, Chapel H, et al. Complement system protein C4 and susceptibility to hydralazine-induced systemic lupus erythematosus. ognia JL.
23. Schaffer JC, Davidson DM, McNiff JM, Bolognia JL. Perinuclear antineutrophilic cytoplasmic antibody-positive cutaneous polyarteritis nodosa associated with minocycline therapy for acne vulgaris. J Am Acad Dermatol. 2001;44:198-206.
24. Matsuura T, Shimizu Y, Fujimoto H, et al. Minocycline-related lupus. Lancet. 1992;340:1553.
25. Lawson TM, Amos N, Bulgen D, Williams BD. Minocycline-induced lupus: clinical features and response to rechallenge. Rheumatology (Oxford). 2001;40:329-335.
26. Elkayam O, Levartovsky D, Brautbar C, et al. Clinical and immunological study of 7 patients with minocycline-induced autoimmune phenomena. Am J Med. 1998;105:484-487.
27. Elkayam O, Yaron M, Caspi D. Minocycline induced arthritis associated with fever, livedo reticularis, and pANCA. Ann Rheum Dis. 1996;55:769-771.
28. Mohan AK, Edwards ET, Cote TR, et al. Drug-induced systemic lupus erythematosus and TNF-alpha blockers. Lancet. 2002;360:646.
29. Bleumink GS, ter Borg EJ, Ramselaar CG, Ch Stricker BH. Etanercept-induced subacute cutaneous lupus erythematosus. Rheumatology (Oxford). 2004;40:1317-1319.
30. Ali Y, Shah S. Infliximab-induced systemic lupus erythematosus. Ann Intern Med. 2002;137:625-626.
31. Day R. Adverse reactions to TNF-alpha inhibitors in rheumatoid arthritis. Lancet. 2002;359:540-541.
32. Chang C, Gershwin ME. Drug-induced lupus erythematosus: incidence, management and prevention. Drug Saf. 2011;34:357-374.
33. Ibuprofen package insert. Livonia, MI: Major Pharmaceuticals; January 2009.
34. Naprelan (naproxen) package insert. Florham Park, NJ: Elan Pharma International; November 2011.
35. Hydrocortisone package insert. Huntsville, AL: Qualitest Pharmaceuticals; April 2008.
36. Methylprednisolone package insert. Parsippany, NJ: Watson Laboratories Inc; December 2003.
37. Prednisone package insert. Columbus, OH: Roxane Laboratories, Inc; September 2009.
38. Plaquenil (hydroxychloroquine) package insert. New York, NY: Sanofi-Synthelabo; October 2000.
To comment on this article, contact rdavidson@uspharmacist.com.





