US Pharm. 2012;37(4):46-48.
Vitiligo is a pigment disorder that involves the skin, the hair, and occasionally the mucous membranes.1,2 All skin types are affected, and in rare cases the iris of the eye can become discolored.3 Vitiligo is progressive and chronic, but some patients spontaneously repigment, although this is uncommon.1 The disorder affects approximately 1% of the U.S. population and 0.1% to 8% of the global population.1 Vitiligo can occur at any age, but the average age of onset is 20 years.1 There is no difference in prevalence between men and women, but women present more often because of cosmetic concerns.
BACKGROUND
Vitiligo is a complex disorder, and its exact causation is unknown. It generally manifests as hypopigmented patches in areas that normally are hyperpigmented, such as the face, hands, and elbows.1 The disorder can be classified as localized, generalized, or universal. Localized vitiligo is restricted to one general area, whereas generalized vitiligo involves more than one area that is symmetrical and usually includes mucous membranes.3,4 Universal vitiligo is the loss of pigment in more than 80% of the skin.1,3
Although the exact mechanism is unknown, the hypopigmentation that characterizes vitiligo has been shown to be due to the absence of functional melanocytes (melanin-producing cells).1,2 Genetic and nongenetic factors are thought to contribute to the pathophysiology of this condition, with 18% to 20% of all cases having a familial trait.1,5
Nongenetic factors theorized to be potentially associated with melanocyte damage include autoimmune defect, melanocyte defect, oxidative-antioxidant system stress, and nerve injury.1,6 Peripheral blood samples from vitiligo patients have been discovered to contain melanocyte-specific autoantibodies and cytotoxic T cells, suggesting an autoimmune disorder. Other research has found that there is an intrinsic abnormality in melanocytes that makes them more susceptible to trauma and environmental changes. (Trauma is believed to precede the onset of vitiligo, causing melanocytic defects as well as nerve injury.) Finally, the epidermis of vitiligo patients has been shown to retain high levels of hydrogen peroxide. This accumulation leads to oxidative stress in the epidermis, resulting in damage to the melanocytes.6 While these are all separate hypotheses, they are all a part of the big picture, and additional research is needed to determine the definitive cause of vitiligo.
Vitiligo, which can be disfiguring, generally presents as round or oval macules with convex margins surrounded by normal skin.1,3 The disorder is most often diagnosed clinically, but the use of a Wood’s lamp may be beneficial for its identification in fair-skinned individuals.1,6 Biopsy also may be useful to verify the absence of melanocytes in the affected area.6 Although vitiligo is generally considered a cosmetic issue, it is associated with the development of autoimmune disorders such as thyroid disease, insulin-dependent diabetes mellitus, Addison’s disease, and pernicious anemia.3,4,6
Quality of life in individuals with vitiligo is often overlooked and underestimated.1,7 The cosmetic nature of the disorder can have a negative impact on a person’s self-esteem, body image, and intimate relationships, as well as cause unnecessary stress.1,3,6 Often, these issues lead people to seek treatment. The treatment of vitiligo can be difficult, and because the exact mechanism is poorly understood, a multifaceted approach is necessary.6,7
TREATMENT OPTIONS FOR VITILIGO
Currently there is no cure for vitiligo, but many options are available for treating vitiliginous lesions.7 Response to treatment is variable, and no single option works for all patients.8 There are three general approaches to treatment: camouflaging depigmented skin, restoring pigmentation, and destroying the remaining pigmentation.
Camouflaging Depigmented Skin
Camouflage therapy is a nonpharmacologic treatment option for vitiligo. It is performed to disguise vitiliginous lesions and to help ease the psychological component of the disease for the patient.9 Products include self-tanners, stains, foundations, and powders.9 Most available foundation and powder products require daily application and easily wear off with sweat and friction, whereas self-tanning products offer a semipermanent option.10 Dihydroxyacetone, the active ingredient in most self-tanners, gives the skin a brown color that lasts up to 10 days.10 Self-tanners should be applied only to the vitiliginous lesions in order to minimize color contrast and should be used sparingly on the elbows, knees, and hands.9 Exfoliation of the skin prior to using the product aids in achieving an even application.9 Sun protection is important, as many self-tanning products do not contain sunscreen.
Vitiliginous lesions lack melanin, the body’s natural sunscreen. For this reason, depigmented lesions have a higher risk of sun damage, photoaging, and skin cancer.11 Sunscreen helps prevent short-term and long-term damage and can minimize the contrast between normal skin and depigmented skin. Patients should use a waterproof sunscreen that protects against both ultraviolet A (UVA) and ultraviolet B (UVB) rays and provides a sun protection factor of at least 15.11 Sunscreen should be reapplied every 90 minutes, and after swimming or sweating.11
Restoring Pigmentation
Phototherapy and Photochemotherapy: One of the oldest vitiligo treatments is UV light, and its use in various forms remains the most common treatment option.7 Phototherapy consists of UVA and narrowband UVB (NB-UVB). Photochemotherapy is the combination of a photoactive chemical (e.g., psoralen, khellin) plus UVA (psoralen plus UVA [PUVA]).7 PUVA requires the patient to take an oral dose of psoralen or to apply it topically via a lotion, cream, or bathing solution prior to UVA exposure. In a single randomized trial comparing oral PUVA with NB-UVB, NB-UVB was more effective, although both agents produced results.12 Side effects of phototherapy include blistering, hyperpigmentation of the surrounding unaffected skin, and skin malignancies (rare).7
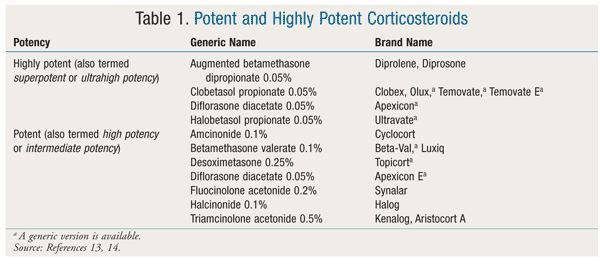
Topical Medications: Generally, the first-line pharmacologic treatment for vitiligo is topical corticosteroids (CSs), which can be applied to most skin lesions.10 The efficacy of CSs is attributed to the drugs’ modulation of immune response. Vitiliginous lesions on the neck and face respond better to CSs than lesions in other areas; however, the use of CSs on the face is limited because of the thinness of the skin and subsequent increased absorption.10 Potent and highly potent products (TABLE 1) are recommended over less potent agents.13,14 Potent CSs such as betamethasone valerate should be tried first, and only if the patient does not respond should high-potency CSs such as clobetasol propionate be used.5 Of note, high-potency CSs should be used for only 1 to 2 months, after which time they should be slowly tapered to a lesser strength.3 In one study, 10% of patients prescribed clobetasol propionate 0.05% achieved near-complete repigmentation, as opposed to 25% of those treated with betamethasone valerate 0.1%.15,16 High-potency CSs should be applied in a thin layer once daily to the depigmented area only, as this will help reduce the risk of side effects such skin atrophy, striae, steroid folliculitis, hypertrichosis, and acne.3,5,10 Systemic absorption is also a concern, especially in patients with thin skin, those with large areas of depigmentation, those with head or neck vitiligo, and children. Potential systemic side effects include insomnia, agitation, weight gain, and adrenal insufficiency.10
Calcineurin inhibitors (CIs)—pimecrolimus 1% and tacrolimus 0.03% or 0.01%—are another effective topical choice.5,10,17 CIs provide immunomodulatory effects without the side effects of CSs, but with similar efficacy. As with CSs, lesions of the head and neck respond best to CIs.5,10,17 The application of hydrocolloid dressings in conjunction with the use of CIs has been shown to enhance repigmentation of resistant arm and leg lesions.18 Common side effects associated with CIs include erythema, pruritus, burning, and irritation. CIs are typically dosed twice daily and are approved for short-term use (2-4 weeks) or intermittent long-term use.10,17 CIs are considered alternatives to topical steroids because of similar efficacy and a better tolerated side-effect profile. Also, CIs may be used in patients with lesions on the face or neck, areas where steroid therapy is less desirable.5,10,17
A third topical immunomodulatory agent is calcipotriene, a vitamin D analogue that enhances the development of melanocytes in vitiligo.10,17,19 Although calcipotriene has been shown to be inferior to topical CSs for monotherapy, it may be an effective adjunctive treatment.20 When calcipotriene is combined with CSs, the rate of repigmentation increases, the delay to repigmentation onset shortens, and there is greater stability of repigmentation compared with either agent alone.20,21 Erythema, dryness, stinging, and burning have been reported as the most common side effects of calcipotriene use.
Complementary Therapy: Patients often look to natural products to prevent or minimize symptoms of various afflictions. Several studies have examined complementary therapies for vitiligo. Ginkgo biloba, an herb used in traditional Chinese medicine, has been studied as a potential therapy because of its antioxidant and immunomodulatory properties.22 In a small double-blind, placebo-controlled study, patients who took G biloba 40 mg three times daily experienced a statistically significant delay in disease progression.23 Also, patients who took G biloba were more likely to have an increase in repigmentation. G biloba has been noted to be well tolerated in most individuals, although some patients report headache, nausea, and bleeding.24 Although G biloba shows some promise in vitiligo treatment, further studies need to be conducted before it can be recommended to patients.
Surgery: Patients who respond poorly to medical treatment may consider undergoing surgery to transplant functional melanocytes to depigmented skin.5 Various surgical techniques are used, but they can be broken down into two main categories: grafting melanocyte-rich tissue and grafting melanocyte cell suspensions.10 It is recommended that surgery be done only on sites that have had no new lesions and no extension of lesions within the past 12 months.3 Also, patients have better outcomes if their vitiligo is more localized, rather than on the extremities. Risks include scarring, graft failure, new lesions at the site of surgery, and hyperpigmentation.10
Destroying the Remaining Pigmentation
Depigmentation: Depigmentation is an option for patients who have vitiligo on more than 50% of their body and who have failed other treatment modalities.5,10 Monobenzone and hydroquinone are topical agents used to induce the death of the remaining melanocytes. These agents may produce results in as little as 1 month, but more often take up to 10 months to achieve desired results.3 Side effects of these medications include erythema, burning, and possible repigmentation.10 Complete depigmentation results in extreme sensitivity to sunlight, so it is important to stress the importance of daily sunscreen use in patients who opt for this treatment.
CONCLUSION
Although vitiligo is not one of the most common dermatologic disorders, it can result in significant cosmetic and psychosocial challenges for individuals who are afflicted with it. Patients suspected to have vitiligo should be referred to a physician for diagnosis and treatment. The pharmacist’s role is to provide information about the application of topical prescription and nonprescription products and cosmetic agents. Pharmacists also have the responsibility to review medication profiles and to provide counseling on the appropriate use of products and the management of potential side effects.
REFERENCES
1. Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a
comprehensive overview. Part I: introduction, epidemiology, quality of
life, diagnosis, differential diagnosis, associations, histopathology,
etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491.
2. Castanet J, Ortonne JP. Pathophysiology of vitiligo. Clin Dermatol. 1997;15:845-851.
3. Kovacs SO. Vitiligo. J Am Acad Dermatol. 1998;38:647-666.
4. Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79:109-116.
5. Gawkrodger DJ, Ormerod AD, Shaw L, et al. Vitiligo: concise evidence based guidelines on diagnosis and management. Postgrad Med J. 2010;86:466-471.
6. Lewis TE. Vitiligo: more than a cosmetic issue. Vitiligo Support
International. https://www.vitiligosupport.org/articles/print.cfm?id=2.
Accessed December 20, 2011.
7. Whitton ME, Ashcroft DM, González U. Therapeutic interventions for vitiligo. J Am Acad Dermatol. 2008;59:713-717.
8. Groysman V, Sami N. Vitiligo treatment & management. Medscape.
September 29, 2011.
http://emedicine.medscape.com/article/1068962-treatment. Accessed
December 30, 2011.
9. Jouary T, DePase A. Camouflage. In: Picardo M, Taïeb A, eds. Vitiligo. Heidelberg, Germany: Springer-Verlag; 2010:423-429.
10. Felsten LM, Alikhan A, Petronic-Rosic V. Vitiligo: a
comprehensive overview. Part II: treatment options and approach to
treatment. J Am Acad Dermatol. 2011;65:493-514.
11. Pacifico A, Leone G, Picardo M. Photoprotection issues. In: Picardo M, Taïeb A, eds. Vitiligo. Heidelberg, Germany: Springer-Verlag; 2010:431-437.
12. Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized
double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A
therapy vs narrowband-UV-B therapy. Arch Dermatol. 2007;143:578-584.
13. Topical corticosteroids. Facts & Comparisons eAnswers [online
database]. St. Louis, MO: Wolters Kluwer Health, Inc; 2012. Accessed
January 12, 2012.
14. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
15. Clayton R. A double-blind trial of 0-05% clobetasol propionate in the treatment of vitiligo. Br J Dermatol. 1977;96:71-73.
16. Kandil E. Treatment of vitiligo with 0-1 per cent betamethasone 17-valerate in isopropyl alcohol—a double-blind trial. Br J Dermatol. 1974;91:457-460.
17. Van Geel N, Boone B, Mollet I, et al. Calcineurin inhibitors. In: Picardo M, Taïeb A, eds. Vitiligo. Heidelberg, Germany: Springer-Verlag; 2010:331-338.
18. Hartmann A, Bröcker EB, Hamm H. Occlusive treatment enhances
efficacy of tacrolimus 0.1% ointment in adult patients with vitiligo:
results of a placebo-controlled 12-month prospective study. Acta Derm Venereol. 2008;88:474-479.
19. Picardo M. Vitamin D analogues. In: Picardo M, Taïeb A, eds. Vitiligo. Heidelberg, Germany: Springer-Verlag; 2010:339-342.
20. Kumaran MS, Kaur I, Kumar B. Effect of topical calcipotriol,
betamethasone dipropionate and their combination in the treatment of
localized vitiligo. J Eur Acad Dermatol Venereol. 2006;20:269-273.
21. Travis LB, Silverberg NB. Calcipotriene and corticosteroid combination therapy for vitiligo. Pediatr Dermatol. 2004;21:495-498.
22. Szczurko O, Shear N, Taddio A, Boon H. Ginkgo biloba for the treatment of vitiligo vulgaris: an open label pilot clinical trial. BMC Complement Altern Med. 2011;11:1-9.
23. Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287.
24. Natural Standard [online database]. Ginkgo biloba.
www.naturalstandard.com/databases/herbssupplements/all/a/. Accessed
December 19, 2011.
To comment on this article, contact rdavidson@uspharmacist.com.





