US Pharm.
2008;33(4)(Oncology suppl):6-19.
ABSTRACT: Multiple myeloma
accounts for approximately 1% of new cancer diagnoses in the United States
each year. The American Cancer Society predicts 19,920 new cases and 10,690
deaths from multiple myeloma in 2008. A major risk factor is exposure to
ionizing radiation. Additional risk factors include exposure to pesticides and
herbicides and a positive family history of the disease. Major complications
of multiple myeloma include bone damage, renal dysfunction, hypercalcemia,
anemia, and neurological effects.
The goal of therapy in patients with
active disease is to induce a complete remission. Remissions may be induced
with dexamethasone, melphalan and prednisone, thalidomide and dexamethasone,
bortezomib or lenalidomide plus dexamethasone, and other regimens. Patients
who are candidates for transplantation may undergo an autologous stem cell
transplant after remission induction. Patients with refractory/relapsed
disease may be treated with bortezomib or lenalidomide plus dexamethasone.
Although multiple myeloma is not curable, median survival is greater than six
to seven years.
Multiple myeloma is a
malignancy characterized by excessive proliferation of plasma cells in the
bone marrow.1 Plasma cells produce five immunoglobulins: IgG, IgA,
IgM, IgD, and IgE. Diseased cells produce one of these immunoglobulins (M
protein) or, on occasion, only the kappa or lambda light chain of the molecule.
1 Excessive cellular proliferation may lead to bone damage,
hypercalcemia, anemia, and neurological complications. Excessive M protein
production can lead to renal dysfunction.
Multiple myeloma accounts for
1% of all cancers in the U.S.2 The American Cancer Society
estimates that 19,920 new cases will be diagnosed in 2008 (11,190 in men and
8,730 in women). Approximately 10,690 people (5,640 men; 5,050 women) will die
from this disease in 2008.3 The mean age at diagnosis is 61 to 62
years.4
Etiology/Risk Factors
The exact cause of
multiple myeloma is unknown. The major risk factor for development is exposure
to ionizing radiation. This may take the form of chronic low-dose exposure
through many diagnostic x-rays over time or a large, single exposure such as
an atomic bomb or proximity to a compromised nuclear reactor.2
Exposure to pesticides and herbicides, including Agent Orange, may also
increase the risk.2 There is also an increased risk in people with
a positive family history of multiple myeloma, especially if two or more
family members have had the disease, and in patients with HIV infections.
Signs and Symptoms
Most patients are
diagnosed based on signs or symptoms of the disease. An early sign is anemia,
which may be due to the presence of malignant cells in the marrow whose
overproduction crowds out the marrow, reducing red cell production, or an
erythropoietin deficiency due to renal damage. Symptoms may include dyspnea,
weakness, and fatigue.
Destruction of bone by myeloma
cells can cause hypercalcemia, which can lead to lethargy, constipation,
nausea, and renal dysfunction. Bone damage may also produce pain and possible
fractures, especially in the spine and proximal long bones such as the femur.
Bone weakness and/or fractures in vertebrae can result in spinal cord
compression. Symptoms of cord compression include back pain, muscle weakness,
and sciatica. Renal dysfunction, seen in about one-third of patients, may be
caused by blockage of the distal renal tubules and/or collecting duct by
intact immunoglobulins or light chains (Bence-Jones proteins). Immune function
may be compromised, increasing the incidence of pneumococcal pneumonia and
herpes zoster infections.2
Diagnosis
A diagnostic
work-up should include a history and physical examination, a CBC with
differential and platelet counts, and assessments of serum calcium, uric acid,
albumin, lactate dehydrogenase, blood urea nitrogen, and creatinine. A bone
marrow aspiration and biopsy should also be performed. A serum and urine
protein electrophoresis should be obtained to identify and quantify a "spike"
in the levels of a monoclonal protein. Serum and urine protein spikes are seen
in 85% and 75% of patients, respectively. Spikes of light chains only are seen
in 10% to 20% of cases. Approximately 3% of patients do not produce urine or
serum proteins and are classified as nonsecretors.5 Additional
tests should include evaluations for quantitative immunoglobulins, serum free
light chains, and immunofixation electrophoresis. An assessment of beta2
-microglobulin is performed to provide an indication of overall tumor burden
and prognosis. A complete skeletal survey should also be performed
Staging
Patients with
multiple myeloma are broadly classified as having smoldering or
active disease. Smoldering myeloma is characterized as asymptomatic
with no end-organ damage or bone lesions and by a serum M-protein level of ?
30 g/L and/or ?10% marrow clonal plasma cells. Active disease is
symptomatic and requires one or more of the following: anemia, hypercalcemia,
renal insufficiency, and lytic or osteopenic bone disease.6 Those
with active disease are further classified according to the stage of their
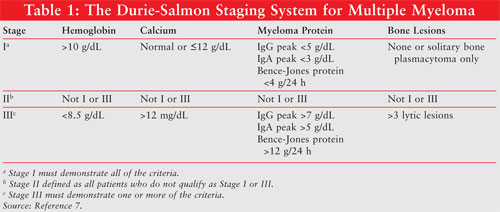
disease. There are two staging systems employed (TABLES 1 and 2
). The Durie-Salmon Staging System employs levels of hemoglobin, calcium, and
myeloma proteins as well as evidence of bone lesions.7 The
International Staging System only uses serum albumin and beta2
-microglobulin levels for staging.8
Management by Stage
Smoldering or
Asymptomatic Stage I Disease:
Patients often have an indolent course that progress slowly over months to
years. The median time to progression to symptomatic disease is two to three
years after diagnosis.9 Therapy may not be necessary at this time.
Observation, consisting of evaluations every three to six months, is performed
until the disease progresses.4 Treatment may become necessary if
the patient develops clinical problems such as renal dysfunction,
hypercalcemia, bone damage, nerve damage, or reduced blood counts.

Active or Symptomatic Disease:
The initial goal of
therapy for patients with active disease is to induce a complete remission.
The European Group for Bone and Marrow Transplant response criteria are the
most commonly employed.10 Responses are classified as complete,
partial, minimal, stable, plateau, relapsed, and progressive disease. A newer
set of response criteria, developed by an international working group, is
under evaluation.11 The most common drug dosing regimens are
presented in TABLE 3.
Once the extent and stage of
disease have been established, patients are evaluated to determine if they are
candidates for a stem cell transplant. Stem cell transplantation is the
standard of care in patients up to age 65 who are transplant candidates.
Patients may be found ineligible for transplantation because of age, physical
condition, comorbidities, or the inability to harvest adequate cells.

Induction Therapy for Transplant
Candidates
Thalidomide:
Thalidomide (Thalomid) is one of several new agents approved for patients
with multiple myeloma. Although the exact mechanism of action is unknown, it
has immunomodulating and antiangiogenesis properties. Major adverse effects
associated with thalidomide include somnolence, fatigue, peripheral
neuropathy, deep venous thrombosis (DVT), constipation, and rash. The
incidence of DVT can be reduced by prophylactic anticoagulant administration
or aspirin. Because of its teratogenic potential, patients must enroll in the
STEPS (System for Thalidomide Education and Prescribing Safety) program before
they can receive the drug. Thalidomide is FDA-approved for use with
dexamethasone in patients with newly diagnosed multiple myeloma.12
In a pivotal trial, Rajkumar
et al randomized 207 patients to dexamethasone or dexamethasone plus
thalidomide.13 Thalidomide was administered at a dose of 200 mg po
daily for four weeks and dexamethasone was administered at a dose of 40 mg po
days 1 to 4, 9 to 12, and 17 to 20 in both arms. Treatment cycles were
repeated every four weeks. There was a significantly higher response in the
thalidomide/dexamethasone arm (63%) compared to dexamethasone alone (41%, P
=.0017). However, the thalidomide arm was associated with significantly more
toxicity including grade 3 DVT or above, neuropathy, rash, and bradycardia.
The incidence of DVT was 17% in the thalidomide arm versus 3% with
dexamethasone alone (P <.001).
Lenalidomide:
Lenalidomide (Revlimid) is an analog of thalidomide.14 The major
adverse effects seen with lenalidomide include marrow suppression,
thromboembolism, rash, and a potential for teratogenesis during pregnancy.
Because of this, patients must be registered and meet all of the conditions of
RevASSIST, a restricted distribution program, before receiving the drug.
14
Although lenalidomide
is currently approved for patients with relapsed/refractory myeloma, it has
been studied as first-line treatment. In one small trial, 34 patients received
lenalidomide 25 mg po daily for 21 days of a 28-day cycle plus dexamethasone
40 mg po days 1 to 4, 9 to 12, and 17 to 20.15 Thirty-one patients
(91%) achieved an objective response, described as a partial response or
better.
A major Phase III trial
randomly assigned patients to lenalidomide 25 mg po daily for 21 days of a
28-day cycle plus high-dose dexamethasone, 40 mg days 1 to 4, 9 to 12, and 17
to 20 (n = 223), or to lenalidomide plus low-dose dexamethasone, 40 mg days 1,
8, 15, 22 (n = 222).16 Lenalidomide plus low-dose dexamethasone
produced a significantly higher one-year overall survival (96.5%) compared to
the high-dose dexamethasone regimen (86%, P <.001). The survival
results were so impressive that an independent monitoring committee
recommended early release of the data and switching all patients to the
low-dose dexamethasone regimen. The low-dose regimen was also associated with
less grade 3 and 4 toxicity including less infection/pneumonia,
thromboembolism, and hyperglycemia. Like thalidomide, patients receiving
lenalidomide should receive anticoagulant prophylaxis.
Bortezomib:
Bortezomib (Velcade) is a proteasome inhibitor that is administered
intravenously. Cancer cells are dependent on proteasome regulatory activities
for proliferation and survival. Inhibition of proteasomes can lead to
disruption of normal cellular homeostasis, which can lead to cell death.
17
Bortezomib was evaluated as
first-line therapy in two small trials. In the first, bortezomib was
administered to 49 patients for up to six cycles. Dexamethasone was added in
cycle 2 or 4, respectively, if a partial or complete response was not
achieved. The overall response rate was 90%. Stem cell transplantation was
performed in 23 patients. The one-year overall survival among transplanted
patients was 90% versus 80% for those not transplanted.18 Therapy
with bortezomib and dexamethasone was well tolerated. The most common grade 2
or higher adverse effects were sensory neuropathy or neuropathic pain (37%),
fatigue (20%), constipation (16%), neutropenia (12%), and nausea (12%). One
patient each developed grade 4 neutropenia and thrombocytopenia.
In the second trial, 48
patients received four cycles of bortezomib plus dexamethasone prior to stem
cell transplantation and achieved a response rate (partial response or higher)
of 66%.19 Adverse effects were reported in 88% of patients (n =
44), 14% of which were reported as serious (n = 7) and required
hospitalization. Most other toxicities were grade 1 or 2 gastrointestinal (GI)
effects. Grade 3 effects included pneumonia, herpes zoster infections,
peripheral neuropathy, GI toxicity, fatigue, infection, rash, and elevation of
alkaline phosphatase and transaminases. One patient experienced a grade 4
reaction consisting of transient intestinal obstruction.
Bortezomib has also been
studied in combination with pegylated doxorubicin and achieved a response of
79% (28% complete response [CR]) in 29 patients who completed therapy; with
thalidomide there was an 82% response (31% CR) reported among 27 patients.
20,21
Induction Therapy for
Nontransplant Candidates
Any induction
regimen available for transplant candidates can also be used in patients who
are not transplant candidates. There are some regimens that can be
administered to nontransplant candidates, but they should not be used in
transplant candidates due to their effects on stem cell reserve. One of these
is melphalan and prednisone (MP).
A meta-analysis of 27
randomized trials involving 6,633 patients that compared MP to other
chemotherapy regimens reported an average response rate of 53% (range 33%-83%)
with MP and a median overall survival of 29 months.22 An advantage
of MP is that it can be administered on an outpatient basis and it is usually
well tolerated. A major disadvantage is that it can decrease the ability to
mobilize stem cells for transplantation. Long-term melphalan use can also lead
to myelodysplasia or leukemia.
Palumbo et al randomly
assigned 255 patients to MP versus melphalan, prednisone, and thalidomide
(MPT). Overall response rates were 76% for MPT and 47.6% with MP. The
respective two-year, event-free survivals were 54% and 27% for MPT and MP (P
=.0006), with three-year overall survivals of 80% and 64% (P =.19),
respectively. Grade 3 and 4 toxicities were significantly more common with MPT
(48% vs. 25%, P =.0002).23 Administration of enoxaparin
reduced the incidence of DVT in the thalidomide arm to 3%.
MPT and MP were also compared
in a three-arm study that randomly assigned 436 patients to MP (191), MPT
(124), or a VAD-based regimen (vincristine, doxorubicin, and dexamethasone)
followed by tandem autologous stem cell transplants (121).24 Median
progression-free survivals (PFSs) were 17.2, 29.5, and 19.0 months in the MP,
MPT, and transplant arms, respectively. PFS was significantly longer with MPT
than MP and with MPT versus the transplant arm (P <.0001), but not with
MP versus the transplant arm. Median overall survivals were 30.3 months with
MP, 38.6 months with the transplant regimen, and were not yet achieved at 56
months with MPT. Median survivals were significantly longer with MPT versus MP
and versus the VAD-based regimen, but not with MP versus the transplant
regimen. Enrollment in the trial was stopped at this interim analysis due to
the superior results with MPT.
Lenalidomide was combined with
MP as induction therapy followed by lenalidomide maintenance therapy in a
trial of 53 newly diagnosed patients.25 The overall response rate
was 85.4%. All patients received aspirin prophylaxis, and thromboembolic
events were seen in three patients, two of whom experienced thromboembolic
events after they discontinued aspirin.
A three-drug combination of
bortezomib, melphalan, and prednisone (VMP) was employed in a Phase I and II
study involving 60 newly diagnosed elderly patients, all of whom were 65 years
or older and almost half of whom were over 75 years of age. Fifty-three
patients completed at least one cycle and achieved an overall response rate of
89%. At a median follow-up of 16 months, the PFS was 91% and the projected
two-year survival was 86%.26
High-Dose Chemotherapy and
Stem Cell Transplantation
After induction,
transplant candidates are reevaluated for response. Responders have their stem
cells harvested in a quantity sufficient to allow for a second transplant, if
needed. The most common transplant is an autologous transplant in which
patients serve as their own donors. The advantages are that there is no risk
of graft versus host disease and the assurance that a suitable donor will be
available.
The results from chemotherapy
induction followed by autologous stem cell transplant studies have been mixed.
Three randomized studies compared conventional-dose chemotherapy to
intermediate- or high-dose chemotherapy plus autologous stem cell
transplantation.27-29 A total of 399 patients received conventional
chemotherapy and 396 received intermediate to high-dose chemotherapy plus
transplantation. All three studies reported significantly higher response
rates, either event-free or PFS, and median overall survival in the transplant
groups. However, two randomized trials failed to demonstrate an improved
response, event-free survival, or overall survival advantage to high-dose
chemotherapy plus autologous stem cell transplantation.30,31
Koreth et al performed a
meta-analysis of nine randomized controlled trials involving 2,411 patients
that compared high-dose therapy and single autologous stem cell
transplantation to standard-dose chemotherapy without transplantation.32
The authors reported that high-dose therapy and autologous transplantation
were associated with a benefit in PFS but not in overall survival.
Relapsed/Refractory Disease
Although
thalidomide is not FDA-approved for this indication, it has been well studied
in patients with relapsed/refractory disease. Glasmacher et al published a
review of 42 Phase II trials of thalidomide alone in 1,629 patients with
relapsed/refractory disease.33 The overall response rate was 29.4%
with a median overall survival of 14 months. Dimopoulos et al evaluated the
activity of thalidomide plus dexamethasone in a population of 44 patients. The
response rate was 55% on an intent-to-treat basis, with a median PFS of 20.3
months and a median overall survival of 12.6 months.34 Palumbo et
al reported a median survival of 55.5 months among 43 patients who received
thalidomide and dexamethasone as a first salvage treatment after autologous
stem cell transplantation.35 In another trial, 62 patients with
relapsed/refractory disease after one chemotherapy regimen achieved a PFS of
17 months and a probability of a three-year survival of 60% with thalidomide
and dexamethasone.36 PFS was 11 months in those who had received
two or more prior chemotherapy regimens.
Bortezomib is FDA-approved for
patients with relapsed/refractory disease.17 In a pivotal study,
669 patients were randomly assigned to bortezomib or high-dose dexamethasone.
37 There was a significantly higher response and one-year survival in
the bortezomib group. Respective response rates were 38% and 18% with
bortezomib versus dexamethasone and respective one-year survivals of 80% and
66% (P =.003). Thirty-seven percent of bortezomib patients had to stop
therapy early due to adverse effects. The most common effects necessitating
discontinuation of therapy were peripheral neuropathy (8%), followed by
thrombocytopenia, nonspecified GI disorders, fatigue, hypercalcemia, and
spinal cord compression (2% each). Adverse effects seen more commonly with
bortezomib in general were GI disorders such as nausea, vomiting, diarrhea,
and constipation, as well as thrombocytopenia and peripheral neuropathy.
Bortezomib alone has been
compared with bortezomib plus pegylated doxorubicin in a randomized Phase III
study involving 646 patients. The overall response rates were 41% for
bortezomib alone and 44% for the combination, an insignificant difference (P
=.43). There was a significant difference in respective median time to
progression (9.3 vs. 6.5 months, P =.000004), median duration of
response (10.2 vs. 7.0 months, P =.0008), and 15-month survival (76%
vs. 65%, P =.03) with the combination compared with bortezomib alone.
38
Lenalidomide is indicated for
use with dexamethasone in patients who have experienced at least one prior
therapy. Two pivotal Phase III randomized trials compared lenalidomide plus
dexamethasone (LD) to dexamethasone alone in patients with refractory/relapsed
disease.39 LD was administered to 345 patients, while
346 received dexamethasone alone. The overall response rates with LD (58% and
61%) were significantly higher than with dexamethasone alone (22% and 23%, P
<.001). The median time to progression was also significantly longer with LD
(13.3 and 15 months) versus 5.1 months in both trials with dexamethasone alone
(P <.000001). Adverse effects were more common in the LD arm, most
notably grades 3 and 4 anemia, neutropenia, and thrombocytopenia.
Thromboembolic complications were more common in the LD arm in one study,
underscoring the need for prophylactic anticoagulation during lenalidomide
therapy.
Maintenance Therapy
Mihelic et al
recently reviewed the literature on maintenance therapy in patients with
myeloma.40 Most of the available data involved the use of
interferon, corticosteroids, or thalidomide. They concluded that the
literature does not support the use of interferon due to lack of improvement
in overall survival and the toxicity associated with interferon
administration, and that there is insufficient data available to recommend
corticosteroids as maintenance therapy. The data on thalidomide maintenance
therapy was not as clear.
There are few major studies
that employed maintenance thalidomide. Those that did had methodological
problems such as small sample sizes, lack of controls, and use of thalidomide
in combination with other treatments, making the contribution of thalidomide
to the outcomes uncertain. Attal et al randomized 597 patients who had
undergone high-dose induction therapy to one of three arms: 1) no maintenance,
2) pamidronate 90 mg/month, or 3) pamidronate 90 mg/month plus thalidomide 100
mg/day.41 The probability of event-free survival three years after
randomization was 36%, 37%, and 52% for arms 1, 2, and 3, respectively; this
was significantly better for those in arm 3 than in either or in other arms of
the study (P <.009). The four-year postdiagnosis probability of
survival was significantly higher for patients in arm 3 (87%) versus no
treatment (77%) or pamidronate alone (74%, P <.04). Subgroup analysis
indicated that patients who did not achieve a partial response and those with
cytogenic abnormality deletion 13 did not benefit from maintenance
thalidomide.
Brinker et al performed a
retrospective review of 112 patients treated with thalidomide and interferon
or observation alone.42 Half of the thalidomide patients received
the drug as maintenance and half after relapse. The overall survival for
patients who received thalidomide at any time after transplantation was 65.6
months versus 44.5 months for those who did not (P =.09). Overall
survival was also significantly better in those who received thalidomide
maintenance therapy (65 months) versus those who received it after relapse (54
months, P =.05). Maintenance thalidomide appears to offer benefit in
select populations; however, it needs further evaluation in controlled
clinical trials.
Prognosis
Data collected by
the National Cancer Institute's Surveillance, Epidemiology, and End Results
(SEER) program from 1973 to 2003 evaluated the survival outcomes in 40,538
patients with multiple myeloma. The average age at diagnosis was 68 years. The
average survival for the entire group was 41 months with a median survival of
24 months. Women experienced a significantly better overall survival than men.
Overall survival was better for younger patients.43
Summary
The initial goal of
therapy for a patient with symptomatic multiple myeloma is to induce a
complete remission with drug therapy. Then, if possible, perform an autologous
stem cell transplant. Several induction regimens have been employed, many of
which involved IV therapy. However, with the advent of two new oral drugs,
oral induction therapy is becoming more common. Oral therapy has also
demonstrated favorable activity in patients with relapsed/refractory disease.
REFERENCES
1. Munshi NC,
Anderson KC. Plasma cell neoplasms. In: DeVita VT Jr, Hellman S, Rosenberg SA,
eds. Cancer Principles and Practice of Oncology. 7th ed. Philadelphia,
PA: Lippincott Williams & Wilkins; 2005:2155-2188.
2. Zaidi AA, Vesole DH. Multiple myeloma: an old disease with new hope for the future. CA Cancer J Clin. 2001;51:273-285.
3. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Multiple Myeloma, V1. 2008. www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Accessed October 2, 2007.
5. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc.2003;78:21-33.
6. International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma, and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757.
7. Durie BGM, Salmon SE. A clinical staging system for multiple myeloma. Cancer. 1975;36:842-854.
8. Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412-3420.
9. Kyle R, Rajkumar S. Multiple myeloma. N Engl J Med. 2004;351:1860-1873.
10. BladÈ J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Brit J Haematol. 1998;102:1115-1123.
11. Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467-1473.
12. Thalomid (thalidomide) package insert. Summit, NJ: Celgene Corporation; February 2007.
13. Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431-436.
14 Revlimid (lenalidomide) package insert. Summit, NJ: Celgene Corporation; March 2007.
15. Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050-4053.
16. Rajkumar SV, Jacobus S, Callander N, et al. Phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2007;25:18S. Abstract LBA8025.
17. Velcade (bortezomib) package insert. Cambridge, MA: Millennium Pharmaceuticals; October 2007.
18. Jagannanth S, Durie BGM, Wolf JL, et al. Long-tern follow-up of patients treated with bortezomib alone and in combination with dexamethasone as frontline therapy for multiple myeloma. Blood. 2006;108. Abstract 796.
19. Harousseau J, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: preliminary results of an IFM phase II study. Haematologica. 2006;91:1498-1505.
20. Orlowski R, Peterson B, Sanford B, et al. Bortezomib and pegylated liposomal doxorubicin as induction therapy for adult patients with symptomatic multiple myeloma: cancer and leukemia group B study 10301. Blood. 2006;108. Abstract 797.
21. Borrello I, Ferguson A, Huff CA, et al. Bortezomib and thalidomide treatment of newly diagnosed patients with multiple myeloma: efficacy and neurotoxicity. Blood. 2006;108. Abstract 3528.
22. Myeloma Trialists' Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16:3832-3842.
23. Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma. Randomized controlled trial. Lancet. 2006;367:825-831.
24. Facon T, Mary J, Harousseau J, et al. Superiority of melphalan-prednisone (MP) + thalidomide (THAL) over MP and autologous cell transplantation in the treatment of newly diagnosed elderly patients with multiple myeloma. J Clin Oncol. 2006;24:18S. Abstract 1.
25. Palumbo A, Falco P, Falcone A, et al. Oral revlimid plus melphalan and prednisone (R-MP) for newly diagnosed multiple myeloma: results of a multicenter phase I/II study. Blood. 2006;108. Abstract 800.
26. Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165-2172.
27. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91-97.
28. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875-1883.
29. Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival in myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052-3057.
30. Segeren CM, Sonneveld P, vander Holt B, et al. Overall and event-free survival are not improved by the use of myeloablative therapy following intensified chemotherapy in previously untreated patients with multiple myeloma: a prospective randomized phase 3 study. Blood. 2003;101:2144-2151.
31. Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myeolme-Autogreffe. J Clin Oncol. 2005;23:9227-9233.
32. Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant . 2007;13:183-196.
33. Glasmacher A, Hahn C, Hoffmann F, et al. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584-593.
34. Dimopoulos MA, Zervas K, Kouvatseas G, et al. Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol. 2001;12:991-995.
35. Palumbo A, Falco P, Ambrosini MT, et al. Thalidomide plus dexamethasone is an effective salvage regimen for myeloma patients relapsing after autologous transplant. Eur J Haematol. 2005;75:391-395.
36. Palumbo A, Bertola A, Falco P, et al. Efficacy of low-dose thalidomide and dexamethasone as first salvage regimen in multiple myeloma. Hematol J. 2004;5:318-324.
37. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487-2498.
38. Orlowski RZ, Nagler A, Sonneveld P, et al. Phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone improves time to progression. J Clin Oncol . 2007;25:3892-3901.
39. Weber D. Two phase III randomized, double-blind, placebo-controlled trials of lenalidomide-dexamethasone vs. dexamethasone for refractory or relapsed multiple myeloma. Presented at: ASCO 41st Annual Meeting; May 14-17, 2005; Orlando, FL.
40. Mihelic R, Kaufman JL, Lonial S. Maintenance therapy in multiple myeloma. Leukemia. 2007;21:1150-1157.
41. Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289-3294.
42. Brinker BT, Waller EK, Leong T, et al. Maintenance therapy with thalidomide improves overall survival after autologous hematopoietic progenitor cell transplantation for multiple myeloma. Cancer. 2006;106:2171-2180.
43. Jawed I, Lee CM, Tward JD, et
al. Survival outcomes for multiple myeloma over three decades: a Surveillance,
Epidemiology, and End Results (SEER) analysis. J Clin Oncol.
2007;25:18S. Abstract 8019.
To comment on this article, contact
rdavidson@jobson.com.






