US Pharm. 2007;32(10)(Oncology suppl):14-26.
ABSTRACT: In the United States, breast
cancer and prostate cancer are the most common types of cancer diagnosed in
women and men, respectively. Due to the hormonal sensitivity of these cancers,
treatment options include not only traditional chemotherapy, radiation, and
surgery but also agents that interfere with hormonal pathways involving either
estrogens or androgens. Applicable drugs employ a variety of mechanisms aimed
at reducing the hormonal influence on these cancers in an effort to enact a
cure or control disease-related symptoms. This article reviews hormonal agents
used in the management of breast cancer and prostate cancer and presents
clinical data supporting their indications.
In 2007, an estimated 180,510
Americans will be diagnosed with breast cancer, and 40,910 will die of the
disease.1 Furthermore, 218,890 men will be diagnosed with prostate
cancer, and 27,050 men will die of the malignancy. Breast cancer and prostate
cancer are the most common cancer diagnoses in women and men, respectively.
Lung cancer is the only malignancy estimated to cause more deaths than either
breast or prostate cancer.1 Breast cancer and prostate both involve
hormones that mediate the growth of cancer cells. Although treatments such as
surgery, radiation, and traditional chemotherapy are used to manage patients
in various stages of these diseases, endocrine-based therapies are commonly
selected for use in patients with hormone-sensitive disease.2,3 A
significant amount of clinical investigation has been performed to determine
the most opportune endocrine agent and when to use it in patients based
on disease and patient characteristics. The application of various
combinations of agents from different classes has also been studied. This
article identifies agents of pharmacologic classes that are used to manage
patients via hormonal processes in either breast or prostate cancer and
describes their indications and mechanisms of action.
Breast Cancer
Since the early 20th century, different staging systems have been used to
assess the extent of disease in patients with breast cancer and other cancers.
Although these staging systems are useful in establishing a prognosis and
selecting appropriate treatment options based on disease characteristics such
as size of the tumor, regional spread to lymph nodes, and presence of distant
metastases, they do not consider such factors as hormonal status.4
Evaluation of tumor biopsy specimens is also useful in identifying cellular
characteristics; this information can help in the selection of therapeutic
agents.
The Role of Estrogen:
Estrogen, a major determinant of female characteristics, mediates normal
growth of breast and uterine tissue.5 Prior to menopause, estrogen
is produced primarily in the ovaries, but also in the adrenal glands and
adipose tissue. However, in postmenopausal women, the adrenal glands and
adipose tissue become the only sites of estrogen production, as the ovaries
lose this function. In premenopausal women, the level of circulating estrogen
is controlled by a negative feedback system involving the hypothal amus,
which secretes gonadotropin- releasing hormone (GnRH), that in turn
stimulates the anterior pituitary gland to secrete follicle-stimulating
hormone (FSH) and luteinizing hormone (LH). These hormones stimulate the
ovaries to produce estradiol and estrone, which are endogenous forms of
estrogen. Circulating estrogen then interacts with target cells containing
receptors for the hormone, such as those in breast, uterine, heart, liver,
bone, and brain tissue.5
Estrogen
is an important factor in breast cancer due to the presence of receptors for
estrogen on the surface of breast cancer cells.5 Estrogen not only
stimulates normal breast cells but also influences the growth of estrogen
receptor–positive (ER+) cancer cells. About 70% to 80% of patients with breast
cancer have cancers that are ER+. Interestingly, as a patient's age at the
time of diagnosis of breast cancer increases, the likelihood for the breast
cancer cells to be ER+ increases. This is important in selecting therapy, as
it suggests that younger women may be less likely to respond to hormonal
therapy targeted at controlling the effects of estrogen on breast cancer cells
than are older women who have passed menopause.5
Selective Estrogen-Receptor Modulators:
Since a variety of tissues have receptors for estrogen, this hormone has
multiple functions. Selective estrogen-receptor modulators (SERMs) provide
antagonistic effects of estrogen on breast cancer cells, but an agonistic
effect on normal cells, such as those in the uterus, bone, and heart.5
This results in the maintenance of some of the positive effects of estrogen
that are otherwise absent with the use of a pure estrogen antagonist. Three
SERMs are currently available in the United States (see Table 1 for
FDA-approved indications). Tamoxifen was the first to be approved, in 1997,
while toremifene (Fareston) and raloxifene (Evista) are more recent additions.
5-7 Only toremifene and tamoxifen are currently approved by the FDA for
the treatment of women with breast cancer.8

Raloxifene has been compared to tamoxifen in its ability to prevent the
occurrence of breast cancer in women at risk of the disease.9 The
National Surgical Adjuvant Breast and Bowel Project's Study of Tamoxifen and
Raloxifene trial involved the monitoring of more than 19,000 postmenopausal
women older than 35 years who were judged to have a five-year predicted breast
cancer risk of at least 1.66% based on the existence of specific risk
factors--such as menarche, age of first live birth, and number of first-degree
relatives (i.e., mother or sister) with breast cancer--in each study
participant. Study participants received either tamoxifen 20 mg daily or
raloxifene 60 mg daily for five years. According to the results, raloxifene
was statistically as effective as tamoxifen in reducing the risk of invasive
breast cancer; however, there was a significantly higher risk in the
occurrence of noninvasive breast cancer in women taking raloxifene than in
those taking tamoxifen.9 Currently, the only approved indication
for raloxifene is in the treatment and prevention of osteoporosis in
postmenopausal women.7
Tamoxifen is considered to be the standard by which other hormonal therapies
can be compared.10 This agent has been demonstrated to be effective
as monotherapy or in combination with traditional chemotherapy to treat
advanced breast cancer and prevent recurrence. It is also effective in
prolonging survival as adjuvant therapy in early-stage disease (i.e., stage I
orII). Tamoxifen has also been shown to lower the incidence of contralateral
breast cancer (i.e., cancer occuring in the breast that was not included in
the original diagnosis).10 Toremifene has been shown to be
effective in extending disease-free survival and overall survival in peri- and
postmenopausal women with ER+ breast cancer.11
Due to
the agonist–antagonist activity of SERMs, there are a variety of side effects
that patients taking these drugs can experience. On the positive side, several
beneficial estrogenic actions are preserved owing to the agonistic
characteristics of the agents in this class. Prior to menopause, rates of
coronary events in women are much lower than in men due to the
cardioprotective effects of estrogen.12 But after menopause, this
protection is lost, and increases in LDL cholesterol and total cholesterol, as
well as a decline in HDL cholesterol, may occur.12 SERMs may help
in the metabolism of cholesterol by reducing levels of LDL cholesterol.5
Although the full effect that SERMs such as tamoxifen may have on managing
coronary disease is unknown, the results have thus far been promising.12
Likewise, the estrogenic effects of SERMs have a protective effect on
maintaining bone density and reducing the risk of bone fractures due to
osteoporosis.5 On the negative side, SERMs can prolong the
discomforting symptoms of menopause, such as hot flashes, vaginal bleeding and
discharge, weight gain, and fatigue.5 Tamoxifen has also been found
to pose a slightly increased risk in the development of endometrial cancer in
women because of the stimulatory effects on uterine tissue. SERMs may also
contribute to the development of thromboembolic events, such as deep venous
thrombosis and pulmonary emboli.5
Luteinizing Hormone–Releasing Hormone Analogs: As mentioned, the
ovaries are the major site of estrogen production in premenopausal women. In
postmenopausal women, only the adrenal glands and adipose tissue produce
estrogen. Therefore, in premenopausal women with breast cancer, surgical
removal of the ovaries, through oophorectomy, or chemical suppression of
estrogen synthesis in the ovaries results in a postmenopausal state. Chemical
suppression can be obtained with the use of luteinizing hormone–releasing
hormone (LHRH) analogs, which are also called GnRH agonists.13 When
administered under chronic conditions, such as with depot injections, these
agents cause the release of LH and FSH. which initially flood the ovaries with
the message to synthesize more estrogen. However, chronic exposure to LHRH
analogs leads to a down-regulation of GnRH receptors and suppression of
gonadotropin (i.e., LH and FSH) secretion. Within three weeks, a reduction in
estradiol levels that mimics those seen in a postmenopausal woman is obtained.
5,13,14 Two LHRH analogs are currently available in the U.S., goserelin
(Zoladex) and leuprolide (Lupron), but only the former is approved for use in
the treatment of breast cancer. As mentioned, to accomplish the constant
exposure to LHRH analogs that facilitates suppression of estrogen synthesis in
the ovaries, these agents are administered as subcutaneous depot injections
given every four weeks or more, depending on the dosage of the injection.
13-15
With regard to the efficacy of
LHRH analogs, one meta-analysis examined these agents: (1) as a substitute for
chemotherapy; (2) in combination with adjuvant hormonal therapy; and (3) in
combination with adjuvant chemotherapy and hormonal therapy.16
Analysis indicated that goserelin 3.6-mg injection given every 28 days for a
period of two years to premenopausal women with ER+ breast cancer resulted in
a statistically equivalent disease-free survival and overall survival,
compared to women who received the CMF (cyclophosphamide, methotrexate,
fluoro uracil) chemotherapy regimen every 28 days for six cycles. Likewise,
goserelin 3.6 mg given every 28 days for at least two years as either
monotherapy or in combination with other hormonal therapy, namely tamoxifen 20
mg daily for five years, resulted in statistically equivalent disease-free
survival. Finally, the data regarding the concomitant use of goserelin and
tamoxifen compared with six cycles of CMF is less convincing due to limited
results. The studies that were performed showed that relapse and overall
survival may be similar, but short-term follow-up and limited patient
recruitment have constrained the ability to make a reasonable conclusion in
this third situation.16
Another
meta-analysis examined the potential benefits of goserelin alone or in
combination with tamoxifen in premenopausal patients with ER+ advanced breast
cancers.17 The meta-analysis also included a study that used the
LHRH analog buserelin, which is not commercially available in the U.S., and
one study that used tamoxifen 30 mg daily. Taking this into account, it was
still observed that the combined endocrine treatment (LHRH analog plus
tamoxifen) resulted in a statistically significantly greater overall survival
over the LHRH analogs used alone. Progression-free survival was also
statistically improved in patients receiving the combination treatment.17
The
benefits observed with LHRH analogs have made them a consideration for use as
second-line therapy in the treatment of breast cancer. The National
Comprehensive Cancer Network (NCCN), a respected source for evidence-based
cancer treatment algorithms, states that an LHRH analog should be considered
for use in premenopausal women who have recurred within at least one year of
previous estrogen receptor antagonist therapy, or in combination with an
estrogen receptor antagonist in women who have not already received an agent
in this pharmacologic class.2 Furthermore, either pre- or
postmenopausal women who have benefited from initial hormonal therapy should
receive additional endocrine therapy, which may include an LHRH analog, at the
time of recurrence.2
Due to the suppression of
estrogen synthesis that is accomplished with use of LHRH analogs, symptoms
associated with estrogen deprivation that are typically observed in
postmenopausal women may occur in patients receiving these agents. These
symptoms may include hot flashes, vaginal bleeding, headaches, depression, and
vaginal dryness.14 Another important concern in patients taking
LHRH analogs after six months is the loss of bone density resulting from the
lack of estrogen. The addition of hormone replacement therapy with an
estradiol/progestin combination product can allow for a continuation of LHRH
analog use beyond six months. This treatment reduces the side effects of low
estrogen levels without compromising the anticancer actions of the LHRH
analog. But using an estrogen in this situation requires additional
investigation and should not be routinely practiced.13,14
Aromatase Inhibitors:
Inhibiting the synthesis of estrogen from nonovarian sites is another method
to control estrogen activity in breast cancer. The adrenal glands produce
androgens that enter the circulation and are converted to estrogen by the
enzyme aromatase.5 Aromatase inhibitors prevent this conversion
from occurring. These agents are useful only in postmenopausal women or in
those women who have had their ovaries removed or suppressed with an LHRH
analog, as they are ineffective against estrogen produced in the ovaries.5
Three aromatase inhibitors are currently available in the U.S.: letrozole
(Femara), anastrozole (Arimidex), and exemestane (Aromasin).18-20
The inclusion of aromatase inhibitors among agents to be considered for
first-line therapy is due in part to results of the Arimidex, Tamoxifen, Alone
or in Combination trial.21 In this study, tamoxifen or anastrozole
alone or the two in combination were randomized to more than 9,000
participants as adjuvant therapy to postmenopausal women with localized breast
cancer. After five years of treatment, anastrozole was associated with a
statistically improved disease-free survival and time to recurrence in all
patients regardless of receptor status. Furthermore, among patients with ER+
disease, significant improvement in disease-free survival and time to
recurrence was observed in patients receiving anastrozole.21
The Breast International Group 1-98 study
compared the use of tamoxifen with that of letrozole as adjuvant endocrine
therapy in postmenopausal women with hormone receptor–positive breast cancer.
22 In this study, 8,010 women were randomized to receive either
letrozole or tamoxifen for five years; letrozole for two years, followed by
tamoxifen for three years; or tamoxifen for two years, followed by letrozole
for three years. Women were assessed for disease-free survival and overall
survival.22 Disease-free survival was found to be statistically
improved in patients receiving letrozole as initial monotherapy, compared to
those receiving tamoxifen. Although the overall survival also favored the
letrozole group, it was not statistically different from that of patients who
received tamoxifen.22
Finally,
in the Italian Tamoxifen Anastrozole Trial, 448 postmenopausal women who had
already received two to three years of tamoxifen therapy were continued on
tamoxifen 20 mg daily or switched to anastrozole 1 mg daily for a total
treatment period of five years.23 Patients were evaluated for
disease recurrence and incidence of death. A significant benefit with regard
to disease recurrence and recurrence-free survival was seen in patients who
were switched to anastrozole versus those who remained on tamoxifen.23
Considering this evidence, the NCCN practice guidelines for breast cancer
suggest that adjuvant endocrine therapy, in the form of a SERM, be considered
for use in hormone receptor–positive women with breast cancer regardless of
their menopausal status, age, or human epidermal growth factor receptor type 2
(HER2/neu) status.2 Although a specific first-line agent is not
recommended by the NCCN guidelines, tamoxifen is suggested as the agent with
the most data to support its use as a first choice. An aromatase inhibitor can
also be considered for use in postmenopausal women as first-line therapy, as
sequential therapy after two years of tamoxifen, or as extended therapy
following 4.5 to six years of tamoxifen. Aromatase inhibitors are
contraindicated in premenopausal women.2
Similarly, the American Society of Clinical Oncology (ASCO) technology
assessment on the use of aromatase inhibitors as adjuvant therapy also states
that these agents should be considered for use in postmenopausal women with
hormone receptor–positive breast cancer.24 The ASCO
assessment suggests that an aromatase inhibitor can be used as first-line
therapy for five years in women who are contraindicated to receive tamoxifen
or as second-line therapy in women who have been taking tamoxifen for two to
three years or five years. The duration of aromatase inhibitor therapy would
depend on how long they had taken tamoxifen.24
Aromatase inhibitors do not act upon estrogen receptors and thus lack many of
the positive effects seen in SERMs, such as improved lipid metabolism and
maintenance of bone density. Due to the lack of action at estrogen receptors,
aromatase inhibitors also lessen the potential for endometrial cancer.9
Although these agents may be less likely to cause symptoms related to
endometrial effects, vaginal pain and dryness can still be problematic.5
Estrogen
Antagonists: In comparison to SERMs, estrogen antagonists have no
agonistic estrogenic activity. Instead, these agents bind to and block
estrogen receptors, leading to their degradation.5,25 Fulvestrant
(Faslodex) is the only drug with this mechanism of action that is available in
the U.S.26,27 Fulvestrant has the ability to reduce the level of
progesterone receptors on breast cancers, an outcome that has not been
observed with the use of SERMs but may help in the control of breast cancer.
27
Two studies have been
conducted to assess the result of using fulvestrant as second-line therapy in
postmenopausal women with progressing advanced breast cancer. One trial
compared fulvestrant 250 mg administered intramuscularly once a month to
anastrozole 1 mg orally daily in patients with locally advanced or metastatic
breast cancer that had progressed during previous endocrine therapy.28
A total of 451 patients were randomized and observed for a median of 14.4
months. Nearly all patients (97% in the fulvestrant group and 98% in the
anastrozole group) had previously received tamoxifen. The remaining patients
had received other available hormonal therapy, so all patients were receiving
study treatment as non–first-line therapy. Analysis showed no statistical
difference in the time to progression among patients treated with either
fulvestrant or anastrozole. With regard to time to treatment failure, again,
no statistical difference was observed. Response rates were also not
statistically different.28
Another study investigated the activity of fulvestrant 250 mg monthly versus
anastrozole 1 mg daily in women with locally advanced or metastatic breast
cancer whose disease had progressed on previous endocrine therapy.29
The 400 patients randomized to each treatment were followed for a median of
16.8 months. Ninety-five percent of fulvestrant participants and 96% of
anastrozole participants had previously received tamoxifen. There was no
significant difference in time to progression, time to treatment failure, or
rate of overall response.29
Together these studies show that fulvestrant offers postmenopausal women with
advanced breast cancer an option that appears statistically equal to that of
an aromatase inhibitor after disease has progressed with tamoxifen therapy.
Side effects that
have been observed with the use of fulvestrant include nausea, diarrhea,
constipation, vomiting, headache, back pain, hot flashes, and pharyngitis.
26
Prostate Cancer
As is the case in breast cancer, therapeutic modalities for prostate cancer
may include surgery, radiation, traditional chemotherapy, and/or hormonal
agents; but androgens take the place of estrogen as the target hormone.
Surgery may include orchiectomy or prostatectomy.30 Susceptibility
of androgen synthesis pathways and androgen receptors to endocrine therapy
helps guide the selection of treatment in this disease. Hormonal therapy for
prostate cancer involves either androgen ablation with LHRH analogs or
blockade of androgen receptors with androgen antagonists (Table 2).
31 These drugs may be helpful in controlling symptoms of disease and
prolonging survival, but androgen-independent disease that is resistant to
hormonal therapy may develop.30,31 Typically, resistance to
hormonal therapy develops after one to two years of treatment.32
The point at which hormone-refractory disease develops may actually be delayed
in those patients who begin therapy at an earlier stage in their disease.
32
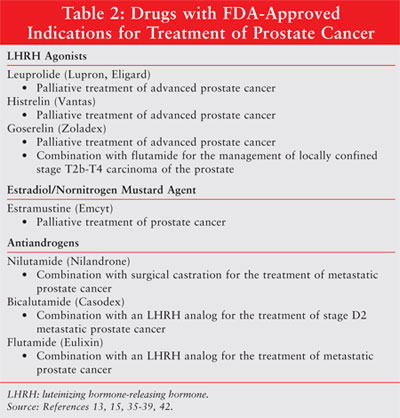
Luteinizing Hormone–Releasing Hormone Analogs: LHRH analogs have
an effect in prostate cancer similar to the one they have in breast cancer;
these drugs reduce the production of testosterone, the active androgen in men.
Unfortunately, as is the case with breast cancer, endocrine therapy is limited
by the lack of androgen-dependent disease in all cases. Although androgen
receptors are found on normal prostate tissue, expression in prostate cancers
is believed to be affected by posttranslational and epigenetic modifications
that may occur with progression of the disease.33 Hormonal therapy
is typically used in combination with local therapy, such as prostatectomy.
34 The intended action of hormonal therapies in prostate cancer is to
eliminate the presence or activity of testicular androgens to the degree
achieved with surgical castration. Typically, clinical outcomes include
palliation of disease-related symptoms by slowing disease progression, since a
cure with hormonal therapy is unlikely to occur. Hormonal therapy also offers
an alternative to orchiectomy, which may be inappropriate for some men due to
side effects such as loss of libido, muscle mass, hot flashes, and
osteoporosis. Many men also find this procedure psychologically unacceptable.
34
LHRH analogs used in prostate cancer treatment include leuprolide (Lupron,
Eligard), histrelin (Vantas), and goserelin (Zoladex).13,15,35,36
Although all of these drugs have the same mechanism of action, a key
pharmaceutical difference between them is the frequency of administration
based on formulated dosage. Lower dosages, such as the goserelin 3.6-mg
product, are given every four weeks; however, the histrelin 50-mg product is
manufactured as a subcutaneous implant that is given once every 12 months.
13,36
Practical guidelines on the treatment of prostate cancer, prepared by the
NCCN, allude to the difficult decision as to when androgen deprivation therapy
should be initiated. According to the guidelines, each case should be
considered on an individual basis. Factors that might encourage use of
androgen deprivation therapy include advanced disease, rate of rising prostate
specific antigen, and ability of patients to tolerate potential side effects
of therapy.3 The NCCN guidelines suggest that an LHRH agonist is
comparable to surgical castration. The concomitant use of an androgen
antagonist for at least the first seven days of LHRH analog use may be helpful
in reducing the incidence or severity of symptoms associated with tumor flare
that occurs with the increase in testosterone production prior to the
therapeutic downregulation of hormone levels.3
Possible side effects of LHRH analog use in men
include hot flashes, sexual dysfunction, and decreased quality of erections.
13,15 Additionally, insertion site reactions may occur with the
administration of histrelin.36 Long-term androgen deprivation can
also lead to increased bone loss, subsequent osteoporosis, and an increased
risk of pathologic fractures.32
Androgen Antagonists: The androgen antagonists block testosterone from
binding to the androgen receptor and inhibit the subsequent stimulus of
cellular activities that include differentiation, secretion, and proliferation.
30,33 Androgen receptor antagonists that are currently available in the
U.S. include bicalutamide (Casodex), flutamide (Eulixin), and nilutamide
(Nilandron).37-39
The Prostate Cancer Trialists' Collaborative Group has evaluated the use of
androgen antagonists in combination with other therapy for prostate cancer
that produces what is referred to as total androgen blockade.40
Findings revealed that the addition of a total androgen blockade extended the
five-year survival of patients by about 2% to 3%, compared to androgen
suppression alone.40 This is a measurable yet small benefit of
using androgen antagonists as a component of combination treatment. The ASCO
2007 Update suggests that despite this small benefit, the use of androgen
antagonists should be considered because of the limited risk of significant
toxicity compared to the use of androgen suppression alone.41
Side effects of
androgen antagonist therapy are often associated with the reduction in
androgen activity and may include gynecomastia, breast tenderness, and hot
flashes.32,37 Hepatic toxicity has also been observed--more so with
the use of flutamide and nilutamide versus the use of bicalutamide.
32,37,38
Conclusion
Both breast and prostate cancers represent a large proportion of newly
diagnosed cancers and cancer-related deaths that occur in women and men in the
U.S. The sensitivity of these cancers to endocrine therapy increases the
number of choices practitioners can select from to treat patients.
Pharmacologic categories include drugs that block the activity of estrogen or
androgens at their respective receptors or drugs that reduce the synthesis of
these hormones. Unfortunately, although these therapies have been found to
provide benefit, questions about the timing and duration of therapy, as well
as its combination with other therapeutic modalities, in various stages of
malignancy still exist.
REFERENCES
1. American Cancer Society. Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007.
2. National Comprehensive Cancer Network. Practice guidelines in oncology. Breast cancer. v.2.2007. Available at: www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Accessed July 22, 2007.
3. National Comprehensive Cancer Network. Practice guidelines in oncology. Prostate cancer. v.2.2007. Available at: www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. Accessed July 26, 2007.
4. Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin. 2006;56:37-47.
5. Bush J. Advances in hormonal therapy for breast cancer. Semin Oncol Nurs. 2007;23:46-54.
6. Fareston [package insert]. Memphis, TN: GTx, Inc.; 2004.
7. Evista [package insert]. Indianapolis, IN: Eli Lilly and Company; 2007.
8. U.S. Food and Drug Administration. Listing of approved oncology drugs with approved indications. Available at: www.FDA.gov/cder/cancer/druglistframe.htm. Accessed July 19, 2007.
9. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
10. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
11. Pagani O, Gelber S, Price K, et al; International Breast Cancer Study Group. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of the International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol . 2004;15:1749-1759.
12. Vogelvang TE, van der Mooren MJ, Mijatovic V, et al. Emerging selective estrogen receptor modulators: special focus on effects on coronary heart disease in postmenopausal women. Drugs . 2006;66:191-221.
13. Zoladex [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2005.
14. Kiesel LA, Rody A, Greb RR, et al. Clinical use of GnRH analogues. Clin Endocrinol (Oxf). 2002;56:677-687.
15. Lupron 7.5 mg [package insert]. Lake Forest, IL: TAP Pharmaceutical Inc.; 2006.
16. Sharma R, Beith J, Hamilton A. Systematic review of LHRH agonists for the adjuvant treatment of early breast cancer. Breast. 2005;14:181-191.
17. Klijn JG, Blamey RW, Boccardo F, et al. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol. 2001;19:343-353.
18. Femara [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2006.
19. Arimidex [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2006.
20. Aromasin [package insert]. New York, NY: Pharmacia & Upjohn Company, Division of Pfizer, Inc.; 2007.
21. Howell A, Cuzick J, Baum M, et al; ATAC Trialists' group. Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60-62.
22. Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747-2757.
23. Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23:5138-5147.
24. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol . 2005;23:618-629.
25. Vergote I, Abram P. Fulvestrant, a new treatment option for advanced breast cancer: tolerability versus exisiting agents. Ann Oncol. 2006;17:200-204.
26. Faslodex [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP.; 2004.
27. Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003;98:229-238.
28. Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396-3403.
29. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386-3395.
30. Hess-Wilson JK, Knudsen KE. Endocrine disrupting compounds and prostate cancer. Cancer Lett. 2006;241:1-12.
31. Culig A, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99:373-381.
32. Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the treatment of prostate cancer. Eur Urol. 2007;51:306-313.
33. Culig Z, Steiner H, Bartsch G, et al. Mechanisms of endocrine therapy-responsive and -unresponsive prostate tumours. Endo Relat Cancer. 2005;12:229-244.
34. Damber JE. Endocrine therapy for prostate cancer. Acta Oncol. 2005;44:605-609.
35. Eligard [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2006.
36. Vantas [package insert]. Lexington, MA: Indevus Pharmaceuticals, Inc; 2007
37. Casodex [package insert]. Wilmington, DE: AstraZeneca Pharmaceutical LP; 2006.
38. AHFS Drug Information. 2007. Available at: online.statref.com.ezproxy.mcphs.edu/TOC/TOC.aspx?FxId=1&SessionId=A89179WXSTLTFVOG. Accessed July 26, 2007.
39. Nilandron [package insert]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; 2006.
40. Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491-1498.
41. Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of the American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596-1605.
42. Emcyt [package insert]. New
York, NY: Pharmacia & Upjohn Company, Division of Pfizer, Inc.; 2006.
To comment on this article, contact
editor@uspharmacist.com.





