US Pharm. 2007;32(7)(Oncology suppl):26-31.
ABSTRACT: Chronic myelogenous
leukemia (CML) is a hematologic malignancy associated with chromosomal
abnormalities, including the Philadelphia chromosome (Ph). The aim of initial
therapies was to reduce the presence of the CML clone using myelosuppressive
remedies. Although these treatments may have provided some level of disease
control, the chance for a cure was minimal. Allogeneic stem cell
transplantation offers an effective cure for patients with CML, but the
morbidity and mortality linked to transplant-related complications, as well as
donor availability, limit this option. The use of interferon-alpha is also an
effective treatment that is limited by tolerability. New targeted therapies,
such as imatinib and dasatinib, which inhibit the enzymatic activity of Ph,
offer patients relatively well-tolerated treatment options with a high
likelihood of response. The objective of this article is to describe the
history of therapeutic options available for CML and the role of current
targeted therapies.
Chronic myelogenous leukemia (CML) is a
disorder of hematopoietic stem cells that results in uncontrolled
myeloproliferation.1 The disease was first described in 1845, and
in 1960, the molecular cause of the disease was determined through the
discovery of the Philadelphia chromosome (Ph), named for the city in which it
was identified. This was the first time that a chromosomal rearrangement could
be linked specifically to the development of a particular cancer.2
Ph is created through the translocation of a section of human chromosome 9
that contains the ABL kinase domain with a specific breakpoint cluster region
(BCR) on chromosome 22. This translocation results in BCR-ABL, a
constitutively active oncogenic tyrosine kinase, which imparts the ability of
cells containing this abnormality to hyperproliferate. Eventually, these cells
are released into the periphery as differentiated leukemic white blood cells.
3,4 Although BCR-ABL is not found in all patients with CML, it is
present in more than 90%, as well as in 10% to 15% of patients with acute
lymphoblastic leukemia (ALL).2 Aside from Ph, other genetic defects
have been observed in over 80% of patients in the blast crisis (BC) phase of
CML, the most aggressive phase of the disease.
Phases of CML
CML primarily
affects the elderly; the median age at diagnosis is 65 years.5 The
diagnosis of CML is often made following the identification of leukocytosis,
prompting further evaluation and subsequent identification of immature blasts
and promyelocytes. Patients are classified into one of three phases based on
the level of clonal expansion of blast cells and promyelocytes in the
peripheral blood and bone marrow. The chronic phase (CP) of CML represents an
early phase with a lower level of myeloproliferation compared to advanced
stages. Of the approximately 4,600 patients diagnosed with CML in the United
States each year, more than 90% are in this phase.5 Although
patients are generally asymptomatic in CP, expansion of the CML clone may lead
to malaise, weight loss, and an enlarged spleen.5 Patients can
remain in CP for three to five years, but progression to the accelerated phase
(AP) signals a shift to a more aggressive form of CML, marked by genetic
instability of the clone and increased volume of blasts and promyelocytes.
2 Physical symptoms representing the transition to AP are often not
apparent to patients. The duration of this phase is generally four to six
months before the progression to BC. In this phase, there are ?30%
blasts in the bone marrow or peripheral blood. Extramedullary sites of blast
cell proliferation are likely. Patients in BC may have complaints of fever,
night sweats, weight loss, anorexia, and fatigue. Splenomegaly is often
present, and patients may experience bone pain and show signs of infection.
Median survival of patients in BC is three to six months.5
Goals of Treatment
Treatment for CML
has changed in recent years due to advances in the understanding of the
molecular basis of the disease and technology, facilitating the discovery of
therapy aimed at inhibiting factors related to pathogenesis and disease
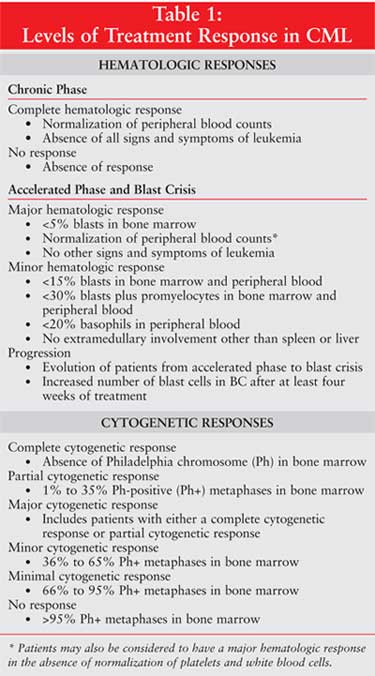
progression. The two basic goals of treatment in patients with CML involve the
hematologic and cytogenetic responses (Table 1). A complete
hematologic response during CP is defined as normalization of peripheral
blood counts and absence of all signs and symptoms of leukemia. The absence of
response is aptly indicated as no response. In the advanced stages of
CML (AP and myeloid BC), a major hematologic response can be a complete
hematologic response if there are less than 5% blasts in the bone marrow, a
normalization of blood counts, and no other signs and symptoms of leukemia. A
major hematologic response may also include patients who present with no
evidence of leukemia but with the absence of normalized platelets or white
blood cell counts. A minor hematologic response is defined as less than
15% blasts in bone marrow or peripheral blood, less than 30% blasts plus
promyelocytes in bone marrow and peripheral blood, less than 20% basophils in
peripheral blood, and no extramedullary involvement other than the spleen or
liver. Progression indicates the evolution of patients from AP to BC or
the increase in number of blasts in patients who are in BC after at least four
weeks of treatment.
For a complete cytogenetic
response, the absence of Ph in the bone marrow is required. A partial
cytogenetic response is the detection of 1% to 35% Ph-positive (Ph+)
metaphases. A minor cytogenetic response is 36% to 65% Ph+ metaphases,
a minimal response is 66% to 95% Ph+ metaphases, and no response
is more than 95% Ph+ metaphases. A major cytogenetic response includes
patients who have achieved either a complete or partial cytogenetic response.
6,7
Evolution of Therapy
Fowler's
Solution: With the identification of CML and a basic understanding of
the disease, pharmacologic therapy began to be employed to control the
disease. Fowler's solution, arsenic trioxide dissolved in potassium
bicarbonate, was first described as a remedy for CML in 1878.8 When
a leukemic patient was given the solution for a 10-week period, leukocytosis
was shown to decrease, then return after discontinuation of therapy, and
respond again after reinstitution of the arsenic. Fowler's solution became a
common treatment for CML and other hematologic malignancies until the turn of
the century, when advances in the understanding of radiation led to the
application of splenic irradiation, which was used as first-line therapy for
CML until the 1950s.8,9 Arsenic solution made a brief return as a
treatment for CML in the 1930s, but reports of toxicity reduced its use.8
Although these early therapeutic options were able to reduce the levels of
blast cells in patients, there was almost no chance for a cure of CML.
Busulfan:The
discovery of busulfan in the 1950s provided another option.10
Typically, busulfan was first administered in intermittent oral doses ranging
up to 6 mg per day. Patients were monitored for a reduction in leukocytosis
during therapy.11 The intermittent pattern of dosage was aimed to
limit toxicity such as skin pigmentation, pulmonary fibrosis, reproductive
disorders, and wasting syndrome. When a patient's blood counts decreased to
levels considered unsafe, the drug was discontinued. If a disease-related rise
in white blood cell count was observed, busulfan was reinitiated. Although
remissions may have lasted several weeks to years in early treatment courses,
the remissions ultimately became shorter with each relapse. When intermittent
therapy was deemed to have lost its effect, continuous busulfan dosing was
prescribed. Other medications were used, such as 6-mercaptopurine, uracil
mustard, and dibromomannitol, but were of limited therapeutic benefit and
never became mainstays of therapy.11,12
Hydroxyurea: In
the 1950s, hydroxyurea was found to have antitumor activity and was tested
against a variety of malignancies, including CML.13 During the
1960s, multiple studies found hydroxyurea to be beneficial in controlling
leukocytosis in patients with CML who relapsed after first-line use of
busulfan. However, patients who responded eventually progressed to the BC
phase of the disease.11 In 1972, clinical studies found hydroxyurea
to be a viable first-line therapy.11 A retrospective review of
patients treated with either busulfan or hydroxyurea published in 1982 found
hydroxyurea to be just as effective as busulfan, with a possible increase in
survival.14 Although busulfan and hydroxyurea offered patients a
chance for improved survival, both agents failed to prevent the inevitable
advancement of CML to the BC phase.
Allogeneic Stem Cell
Transplantation (SCT): The goal of treatment for CML began to shift in
the 1970s with allogeneic SCT. This procedure offered a means to eradicate Ph
through various myelosuppressive and immunosuppressive treatment combinations,
termed conditioning regimens, of chemotherapy and radiation.15,16
Thus, a realistic chance for a cure became available. In allogeneic SCT,
antigenetically matched donor stem cells are transfused into patients after
the myelosuppressive conditioning regimen is given. The donor cells then
replace and rebuild the patient's hematopoietic system and mount a complex,
immune-mediated reaction against remaining malignant cells.
Allogeneic SCT is most
successful early in the course of disease; hence, patients undergoing
transplantation in CP have an improved chance of survival when compared to AP
patients, and AP patients fair better than patients in BC.17
Allogeneic SCT is highly effective in achieving cytogenetic and hematologic
responses; the relapse rate after a sibling SCT is less than 20% for patients
transplanted in CP.18 The five-year disease-free survival rate in a
similar group of patients who received stem cells from unrelated donors (which
is considered to carry a higher risk of treatment-related complications) was
also between 80% and 90%.5 Therefore, it is important that SCT be
conducted as early in the progression of CML as possible. Current practice
continues to maintain this understanding, and allogeneic SCT remains a common
treatment modality.18 Although SCT offers a possible cure for CML,
this option is limited by donor availability and treatment complications, such
as hepatic veno-occlusive disease, graft-versus-host disease, and potentially
fatal infections.
Interferon-Alpha:
The limitations of SCT have led to the evaluation of other therapeutic options
with the potential outcome of a cure. In the 1980s, interferon-alpha offered
patients with CP-CML a possibility for hematologic and cytogenetic responses
with the elimination of Ph.19,20 Currently, interferon-alpha
(Roferon-A) is approved for the treatment of CML in CP with an initial dose of
3 MU daily for three days, followed by an increase to 6 MU daily for three
days, and then an increase to the target dose of 9 MU daily for the duration
of treatment.21 Common side effects of interferon-alpha include
flu-like symptoms, such as fever, fatigue, myalgia, chills, arthralgia, and
headache, as well anorexia, depression, nausea and vomiting, and diarrhea.
21
In the mid-1990s, the addition
of monthly courses of low-dose cytarabine (20 mg/m2 per day for 10
consecutive days repeated monthly) was found to significantly improve survival
over interferon-alpha alone when given to patients with CP-CML.22
Patients receiving interferon-alpha plus cytarabine were found more likely to
achieve a hematologic response at six months and a major cytogenetic response
at 24 months, compared to interferon-alpha alone. Furthermore, patients given
the combination who achieved a partial or complete cytogenetic response were
found to have a longer overall survival, compared to those with a minor or no
response, thus demonstrating the importance of a cytogenetic response to
therapy. Additional studies evaluating the ability of interferon-alpha and
low-dose cytarabine to achieve hematologic and cytogenetic responses and
improve survival in patients with CP-CML have confirmed these results.
23,24 However, toxicity associated with interferon-alpha often limits
the utility of this agent. Side effects often lead to intolerance of
interferon and discontinuation of therapy.22
Targeted Therapy
Imatinib
(Gleevec): In the 1990s, based on the hypothesis that selective
inhibition of BCR-ABL tyrosine kinase activity might be effective in the
treatment of Ph+ leukemias, along with a better appreciation of the
interactions of the ATP-binding site of BCR-ABL, several small molecules with
therapeutic potential were developed.25 One of these compounds
(originally named CGP-57148 and then STI-571) was found to inhibit the BCR-ABL
kinase containing clones in vitro and led to their destruction via apoptosis.
25-27 This compound represented the first targeted therapy created
specifically to inhibit the molecular abnormality believed responsible for
pathogenesis. In 2001, STI-571, now called imatinib, was approved for
use based on studies that found the drug provided Ph+ CML patients with a safe
and effective treatment option.28 Phase I studies showed that
imatinib produced major cytogenetic and hematologic responses in patients who
had not responded to previous treatment with interferon-alpha due to relapse
or intolerance of side effects.29 Imatinib was even found to
benefit patients who did not respond to hydroxyurea, busulfan, or interferon
plus cytarabine.29 This study, among others, provided strong
examples of how significant a role targeted therapy could have in the possible
cure for CML.30
The efficacy of imatinib in
the treatment of newly diagnosed patients with CP-CML who have not received
treatment has also been demonstrated in comparison to the preexisting standard
of interferon plus low-dose cytarabine.31 A randomized phase III
trial of 1,106 patients enrolled within six months of their diagnosis with
CP-CML found that those who received imatinib were statistically more likely
to achieve a complete hematologic and cytogenetic response, compared to
patients who received interferon plus cytarabine. Furthermore, after 12 months
of treatment, patients who received imatinib were less likely to have disease
progression. Although survival rates were comparable for the two groups, the
quality of life related to adverse effects of treatment was better in patients
taking imatinib.31
Imatinib is approved for use
in adult and pediatric patients with newly diagnosed Ph+ CML in CP, as well as
in Ph+ patients in any phase of CML after therapeutic failure of interferon.
Imatinib is also indicated for use in pediatric patients with Ph+ CML in CP
who have recurred after SCT or are resistant to interferon therapy.32
Imatinib is available in 100- and 400-mg tablets. The recommended dosage is
400 mg per day in adult patients in CP, and 600 mg daily in adult patients in
AP or BC. The pediatric daily dose in newly diagnosed patients is 340 mg/m
2 (not to exceed 600 mg per day). Dosing in children with disease
recurrence in CP after SCT or interferon resistance is 260 mg/m2
per day.32 Common side effects in patients taking imatinib include
fluid retention, nausea, muscle cramps, musculoskeletal pain, diarrhea, rash,
fatigue, headache, and joint pain. Cytopenias may also occur, especially at
doses greater than 600 mg daily, and are more likely to be severe in advanced
stages of CML.32
Imatinib clearly
revolutionized the treatment of CML, but limitations in its spectrum of
activity have become apparent. Although patients with CML obtain durable
cytogenic responses to imatinib, relapses have been observed, especially in
patients who begin treatment during AP and BC.6,33,34 Relapse
during treatment with imatinib is often found to be related to resistance
caused by amino acid point mutations occurring within the kinase domain of
BCR-ABL. In fact, these point mutations at over 40 different amino acid
positions account for acquired resistance in 50% to 90% of patients.5,7
The degree of resistance to imatinib when started in CP has been predicted to
occur at a rate of 4%; this rate is higher in advanced stages of CML.35
Resistance to imatinib occurs due to the disruption of critical interactions
between imatinib and BCR-ABL that occur from changes in amino acid sequences.
33,36 The result is the reactivation of BCR-ABL, which leads to
downstream signaling of malignant processes and proliferation of clones
containing the abnormal kinase.34 Other proposed mechanisms of
resistance to imatinib include amplification of the BCR-ABL gene,
overexpression of BCR-ABL mRNA, increased efflux of imatinib via p
-glycoprotein–mediated actions, and activation of additional proteins such as
those belonging to the SRC family of kinases.34,37
Dasatinib (Sprycel):
This SRC family kinase inhibitor is structurally distinct from imatinib.33
Dasatinib was approved by the FDA for use in 2006 and offers promise in the
treatment of CML and Ph+ ALL because of its activity against many of the
mutant forms of BCR-ABL that are resistant to imatinib. This broader spectrum
of activity is due to the ability of dasatinib to bind to the inactive form of
BCR-ABL, similarly to imatinib, but also to the active conformation of the
protein.6 This expanded activity is due to dasatinib's advantage of
less sensitive molecular interactions and subsequent fewer restrictions
required for binding.38
Clinical evaluation of
dasatinib has been performed in patients who have either relapsed after
responding to imatinib or were unable to tolerate the drug due to side
effects. In an early phase I, nonrandomized study examining doses of dasatinib
ranging from 15 mg daily to 120 mg twice daily in adult patients with CML or
Ph+ ALL who were resistant to or could not tolerate treatment with imatinib,
44% achieved a complete hematologic response and 21% a major cytogenetic
response.6 Notably, 81% of patients who had a major cytogenetic
response to imatinib also attained this response taking dasatinib before they
relapsed. In addition, many patients who did not have a cytogenetic response
with imatinib cytogenetically responded to dasatinib. Typically, doses of at
least 50 mg daily were required for the hematologic responses, while higher
doses were needed for major cytogenetic responses. The duration of responses
in patients in CP or AP was between two and 19 months, and 43% of patients in
myeloid BC were still in major hematologic response for five to 12 months at
the time the data were originally published.6 One observed
advantage of dasatinib over imatinib is the activity in patients with
imatinib-resistant mutations. Of the 60 patients who had mutations in BCR-ABL
at baseline, dasatinib was able to invoke a hematologic or cytogenetic
response in all except those patients carrying the T315I mutation. These
results showed that dasatinib offers strong antileukemic activity in patients
with CML or Ph+ ALL, regardless of phase or BCR-ABL genotype, who had been
unable to continue treatment with imatinib.6
The START trials are a series
of comprehensive studies to further ascertain the potential for dasatinib in
the treatment of Ph+ leukemias.39,40-43 The phase II START trial
involved five separate arms to evaluate the effects of dasatinib in patients
in different stages of CML or Ph+ ALL who were resistant or intolerant to
imatinib treatment. In the open-label, nonrandomized START-C, -A, -B, and -L
arms, patients were initiated on 70 mg of dasatinib orally twice daily. The
dosage was escalated to 90 or 100 mg twice daily in patients who were
determined to have a poor initial response, or dosage was decreased to 50 and
40 mg twice daily if persistent drug-related toxicity was observed. In each
arm, patients taking dasatinib achieved hematologic and cytogenetic responses.
Notably, patients in CP were more likely to achieve responses than patients in
either AP or BC.39-42
START-R, the final arm, is the
first randomized, comparative trial of dasatinib versus imatinib.43
Patients with CML in CP that were deemed resistant to 400 to 600 mg of
imatinib were randomized to receive either 70 mg of dasatinib twice daily or
high-dose imatinib at 800 mg per day. The primary endpoint was major
cytogenetic response after 12 weeks of therapy. Preliminary data on the first
36 patients are available. Of these patients, 22 received dasatinib and 14
received imatinib. Toxic effects requiring dose reduction occurred in eight
(36%) of the patients receiving dasatinib and in one patient (7%) in the
imatinib arm. Regarding response to therapy, 21 patients (95%) who received
dasatinib and 13 patients (93%) taking imatinib achieved a complete
hematologic response. At 12 weeks, seven patients (32%) taking dasatinib and
one patient (7%) taking imatinib attained the primary outcome of a major
cytogenetic response. Thirteen patients (two taking dasatinib and 11 taking
imatinib) required therapeutic crossover due to adverse effects.43
Although still preliminary, these results appear promising for patients with
resistance to doses of imatinib of 600 mg daily or less.
Dasatinib is approved by the
FDA for use in the treatment of patients with chronic, accelerated, or myeloid
or lymphoid blast phases of CML or with Ph+ ALL that has developed resistance
or intolerance to previous therapy.44 The recommended starting dose
is 140 mg per day administered orally as 70-mg doses twice daily, morning and
evening without regard to meals. Dasatinib is available as oral tablets that
should be swallowed whole, not crushed, cut, or chewed. The
dasatinib dosage may be increased or decreased in 20-mg increments based on
disease response and tolerability. It is available in three tablet strengths:
20, 50, and 70 mg.44
Resistance created by BCR-ABL
mutations appears to be a limitation of all known tyrosine kinase inhibitors.
Even dasatinib, which seems to have the broadest spectrum of activity, is not
effective against the T315I mutation. However, the most challenging goal in
the treatment of CML may reside in elimination of the pathologic stem cells.
The quiescent nature of these cells may make them inherently resistant to
kinase inhibitors.45,46 In vitro analysis has shown dasatinib to
have only a moderate ability to eradicate cells within the stem cell
compartment. Combining tyrosine kinase inhibitors with drugs that work by
different mechanisms may ultimately be the answer, potentially offering a
higher chance for a cure through the targeting of multiple malignant functions
in CML cells.47-49 Kinase-independent pathways may also have an
important role in the pathology of CML, allowing cells to proliferate despite
adequate exposure to tyrosine kinase inhibitors. This may also explain why
patients in more advanced stages of CML are less likely to respond to
treatment with a tyrosine kinase inhibitor.50
Nilotinib (AMN107):
Nilotinib, a third selective tyrosine kinase inhibitor, is currently still
under investigation, but clinical study has been performed to evaluate its
safety and tolerability, as well as antileukemic activity.51 The
phase I dose-ranging study evaluated 106 patients with Ph+ imatinib-resistant
CML in various phases who were assigned to receive one of nine doses (daily
dose of 50, 100, 200, 400, 600, 800, or 1,200 mg; twice-daily doses of 400 or
600 mg). No dose-limiting side effects were observed for doses up to 600 mg
daily, suggesting this to be the maximum tolerated dose. Hematologic responses
were observed in all three phases of CML (BC, 39%; AP, 72%; CP, 92%). Major
cytogenetic responses occurred as well (BC, 18%; AP, 20%; CP, 53%). Nilotinib
is active in patients with all observed variants of BCR-ABL except the T315I
mutation.51 Therefore, nilotinib, similar to dasatinib,
appears to be a second-line therapy for patients who have relapsed or not
tolerated initial therapy with imatinib.
Conclusion
The evolution in
management for CML has taken several steps over the past 150 years. Some of
these steps, such as the tyrosine kinase inhibitors, represent revolutionary
innovations based on the increased understanding of molecular mechanisms of
CML. These selective therapies offer more safety and reliability than existing
options. Future advances in the treatment of CML will determine how these
novel therapies best fit into existing niches or even alter treatment
patterns. For example, dasatinib and nilotinib are currently used as
second-line targeted therapy after imatinib failure or intolerance. The
question remains as to whether these newer tyrosine kinase inhibitors are as
effective as existing first-line therapy. Similarly, the development of
additional tyrosine kinase inhibitors that provide enhanced activity against
all BCR-ABL forms would be helpful to eliminate existing gaps, such as the
T315I mutation. The investigational agent MK-0457 (VX-680) is an aurora kinase
inhibitor with tyrosine kinase–inhibiting activity, which has been observed in
vivo to provide activity against this particular form of BCR-ABL.52
It may be possible to use multiple tyrosine kinase inhibitors concomitantly to
provide broader coverage and slow the development of resistance. Similarly,
due to the existence of chromosomal changes that occur in CML in addition to
BCR-ABL, more drugs will likely be developed that will help provide a
multipronged effort on different targets involved in the pathogenesis of the
CML.
References
1. Garcia-Manera G,
Faderl S, O'Brien S, et al. Chronic myelogenous leukemia: a review and update
of therapeutic strategies. Cancer. 2003;98:437-457.
2. Wong S, Witte ON.
The BCR-ABL story: bench to bedside and back. Ann Rev Immunol. 2004
;22:247-306.
3. Lombardo LJ, Lee FY,
Chen P, et al. Discovery of
N-(2-Chrolo-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
(BMS0354825), a dual Src/Abl kinase inhibitor with potent antitumor activity
in preclinical assays. J Med Chem. 2004;47:6658-6661.
4. Tokarski JS, Newitt
JA, Chang CYJ, et al. The structure of dasatinib (BMS-354825) bound to
activated ABL kinase domain elucidates its inhibitory activity against
imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790-5797.
5. Quintas-Cardama A,
Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clin
Proc. 2006;81:973-988.
6. Talpaz M, Shah NP,
Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia
chromosome-positive leukemias. New Engl J Med. 2006;354:2531-2541.
7. Quintas-Cardama A,
Kantarjian H, Jones D, et al. Dasatinib (BMS-354825) is active in Philadelphia
chromosome positive chronic myelogenous leukemia after imatinib and nilotinib
(AMN-107) therapy failure. Blood. 2007;109;497-499.
8. Waxman S, Anderson
KC. History of the development of arsenic derivatives in cancer therapy.
Oncology. 2001;6(suppl 2):3-10.
9. Kalidas M,
Kantarjian H, Talpaz M. Chronic myelogenous leukemia. JAMA.
2001;286:895-898.
10. Galton DA. Myleran
in chronic myeloid leukaemia: results of treatment. Lancet.
1953;264:208-213.
11. Sokal JE. Current
concepts in the treatment of chronic myelocytic leukemia. Ann Rev Med.
1973;24:281-288.
12. Canellos GP, Young
RC, Nieman PE, et al. Dibromomannitol in the treatment of chronic granulocytic
leukemia; a prospective randomized comparison with busulfan. Blood.
1975;45:197-203.
13. Kennedy BJ.
Hydroxyurea therapy in chronic myelogenous leukemia. Cancer.
1972;29:1052-1056.
14. Bolin RW, Robinson
WA, Sutherland J, et al. Busulfan versus hydroxyurea in long term therapy of
chronic myelogenous leukemia. Cancer. 1982;50:1683-1656.
15. Fefer A, Cheever
MA, Thomas ED, et al. Disappearance of Ph1-positive cells in four patients
with chronic granulocytic leukemia after chemotherapy, irradiation, and marrow
transplantation from an identical twin. New Engl J Med.
1979;300:333-337.
16. Clift RA, Buckner
CD, Thomas ED, et al. Treatment of chronic granulocytic leukaemia on chronic
phase by allogeneic marrow transplantation. Lancet. 1982;2:621-623.
17. Speck B, Bortin MM,
Champlin R, et al. Allogeneic bone-marrow transplantation for chronic
myelogenous leukaemia. Lancet. 1984;1;665-668.
18. Grigg A, Hughes T.
Role of allogeneic stem cell transplantation for adult chronic myeloid
leukemia in the imatinib era. Biol Blood Marrow Transplant.
2006;12:795-807.
19. Talpaz M, McCredie
KB, Marligit GM, et al. Leucocyte interferon-induced myeloid cytoreduction in
chronic myelogenous leukemia. Blood. 1983;62:689-692.
20. Talpaz M,
Kantarjian HM, McCredie K, et al. Hematologic remission and cytogenetic
improvement induced by recombinant human interferon alpha A in chronic
myelogenous leukemia. New Engl J Med. 1986;314:1065-1069.
21. Hoffman-La Roche,
Inc. Roferon package insert. Nutley, NJ, August 2006.
22. Guilhot F, Chastang
C, Michallat M, et al. Interferon alfa-2b combined with cytarabine versus
interferon alone in chronic myelogenous leukemia. New Engl J Med.
1997;337:223-229.
23. Baccaroni M, Rosti
G, de Vivo A, et al. A randomized study of interferon-alpha versus
interferon-alpha and low dose arabinosyl cytosine in chronic myeloid leukemia.
Blood. 2002;99:1527-1535.
24. Kantarjian HM,
O'Brien S, Smith TL, et al. Treatment of Philadelphia chromosome-positive
early chronic phase chronic myelogenous leukemia with daily dosing of
interferon alpha and low dose cytarabine. J Clin Onc. 1999;17:284-292.
25. Buchdunger EA,
Zimmermann J, Mett H, et al. Inhibition of the Abl protein tyrosine kinase in
vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res.
1996;56:100-104.
26. Druker BJ, Tmura S,
Buchdunger, E, et al. Effects of a selective inhibitor of the Abl tyrosine
kinase on the growth of BCR-ABL positive cells. Nat Med. 1996;2:561-566.
27.
Gambacerti-Passerini C, le Coutre P, Malogni L, et al. Inhibition of the ABL
kinase activity blocks the proliferation of BCR-ABL positive leukemic cells
and induces apoptosis. Blood Cells Mol Dis. 1997;23(19):280-294.
28. U.S. FDA. FDA
approves Gleevec for leukemia treatment. FDA News. May 10, 2001.Print
Media #301-827-6242. Available at:
www.fda.gov/bbs/topics/news/2001/new00759.html. Accessed May 30, 2007.
29. Druker BJ, Talpaz
M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL
tyrosine kinase in chronic myeloid leukemia. New Engl J Med.
2001;344:1031-1037.
30. Druker BJ, Sawyers
CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL
tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute
lymphoblastic leukemia with the Philadelphia chromosome. New Engl J Med
. 2001;344:1038-1042.
31. O'Brien SG, Guilhot
F, et al. Imatinib compared with interferon and low-dose cytarabine for newly
diagnosed chronic phase chronic myeloid leukemia. NEJM.
2003;348:994-1004.
32. Novartis
Pharmaceuticals Corporation. Gleevec package insert. East Hanover, NJ, Nov.
2006.
33. Bradeen HA, Edie
CA, O'Hare T, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825),
and nilotinib (AMN107) in an N-ethyl-N nitrosourea (ENU)-based mutagenesis
screen: high efficacy of drug combinations. Blood. 2006;108:2332-2338.
34. Gorre ME, Mohammed
M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by
BCR-ABL gene mutation or amplification. Science. 2001;293:876-880.
35. Jabbour E, Cortes
J, Kantarjian HM, et al. Allogeneic stem cell transplantation for patients
with chronic myeloid leukemia and acute lymphocytic leukemia after BCR-ABL
kinase mutation-related imatinib failure. Blood. 2006;108:1421-1423.
36. von Bubnoff N,
Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to
clinical resistance of Philadelphia-chromosome-positive leukaemia to STI-571:
a prospective study. Lancet. 2002;359:487-491.
37. Yokota A, Kimura S,
Masuda S, et al. INNO-406, a novel BCR-ABL/lyn dual tyrosine kinase inhibitor
suppresses the growth of Ph+ leukemia cells in the central nervous system and
cyclosporine A augments its in vitro activity. Blood. 2007;109:497-499.
38. Copland M, Hamilton
A, Elrick LJ, et al. Dasatinib (BMS-354825) targets earlier progenitor
population than imatinib in primary CML but does not eliminate the quiescent
fraction. Blood. 2006;107:4532-4539.
39. Hochhaus A,
Baccarini M, Sawyers C, et al. Efficacy of dasatinib in patients with chronic
phase Philadelphia chromosome positive CML resistant or intolerant to
imatinib: first results of the CA-180013 ‘START-C' phase II trial.
Blood. 2005;106. Abstract 41.
40. Ottmann OG,
Martinelli G, Dombert H, et al. A phase II study of dasatinib in patients with
chronic myeloid leukemia (CML) in lymphoid blast crisis of Philadelphia
chromosome positive acute lymphoblastic leukemia (Ph+ALL) who are resistant or
intolerant to imatinib: the ‘START-L' CA180015 study. Blood.
2005;106. Abstract 42.
41. Guilhot F, Apperley
JF, Shah N, et al. A phase II study of dasatinib on patients with accelerated
phase chronic myeloid leukemia (CML) who are resistant or intolerant to
imatinib: first results of the CA180005 ‘START-A' study. Blood.
2005;106. Abstract 39.
42. Talpaz M, Rousselot
P, Kim DW, et al. A phase II study of dasatinib in patients with chronic
myeloid leukemia (CML) in myeloid blast crisis who are resistant or intolerant
to imatinib: first results of the CA180006 ‘START-B' study.
Blood. 2005;106. Abstract 40.
43. Shah NP, Rousselot
P, Pasquini N, et al. Dasatinib (D) vs. high dose imatinib (IM) in patients
(pts) with chronic phase chronic myeloid leukemia (CP-CML) resistant to
imatinib: results of CA180017 START-R randomized trial. J Clin Onc.
2006;ASCO Annual Meeting Proceedings;24(18s). Abstract 6507.
44. Bristol-Myers
Squibb Company. Sprycel package insert, Princeton, NJ, June 2006.
45. Copland M, Hamilton
A, Baird JW, et al. Dasatinib (BMS-354825) has increased activity against
BCR-ABL compared to imatinib in primary CML cells in vitro, but does not
eradicate quiescent CML stem cells. Blood. 2005;106. Abstract 695.
46. Holyaoke TL. Punish
the parent not the progeny. Blood. 2005;105:1862-1866.
47. Copland M, Hamilton
A, Allan EK, et al. BMS-214662 targets quiescent chronic myeloid leukaemia
stem cells and enhances the activity of both imatinib and dasatinib
(BMS-354825). Blood. 2005;106. Abstract 693.
48. Lee FY, Wen ML,
Camuso A, et al. Quiescent chronic myeloid leukemia (CML) cells are resistant
to BCR-ABL inhibitors not preferentially sensitive to BMS-214662, a
farnesyltransferase inhibitor (FTI) with unique quiescent-cell selective
cytotoxicity. Blood. 2005;106. Abstract 1993.
49. Peng C, Hu Y, Lee
FY, et al. Targeting chronic myeloid leukemia (CML) stem cells by BMS-214662
in mice. Blood. 2005;106. Abstract 2861.
50. Li S, Hu Y,
Swerdlow S, et al. Targeting BCR-ABL kinase activity independent signaling
pathways and leukemia stem cells is essential for curative therapy of
Philadelphia chromosome positive (Ph+) leukemia. Blood. 2005;106.
Abstract 199.
51. Kantarjian H, Giles
F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia
chromosome-positive ALL. New Engl J Med. 2006;354:2542-2551.
52. Giles F, Cortes J,
Jones D, et al. MK-0457, a novel kinase inhibitor, is active in patients with
chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL
mutation. Blood. 2007;109:500-502.






