US Pharm. 2007;32(4)(Oncology suppl):6-18.
Erythropoiesis, or the production of erythrocytes (i.e., red blood cells), begins when the kidney senses low levels of oxygen in the blood and releases erythropoietin from the peritubular cells. Erythropoietin binds to receptors on erythroid progenitors (i.e., red blood cell precursors such as burst-forming unit erythroid and colony-forming unit erythroid) in bone marrow and stimulates red blood cell proliferation and maturation.1 Reticulocytes (i.e., immature erythrocytes) lose their nucleus and become mature red blood cells in about seven days.2 Red blood cells are produced in the marrow of the skull, vertebrae, pelvis, and proximal long bones.3 Approximately 250 trillion red blood cells are produced each day (i.e., two million per second). These cells make up 99% of the formed elements, or cellular portion, of the blood.
A typical erythrocyte contains about 280 million hemoglobin (Hgb) molecules, with each erythrocyte carrying four heme groups. The heme group delivers oxygen, which is essential for cellular respiration, and then removes waste products such as carbon dioxide from the tissues.2 After an average life cycle of 120 days, red blood cells are engulfed by phagocytes and destroyed primarily in the liver and spleen. The heme component of the cell is then excreted into the bilirubin.4
When the normal cycle of red blood cell production and reabsorption is disrupted, anemia can occur. Anemia is a deficiency in the oxygen-carrying capacity of the blood and has been defined as a low number of red blood cells or a Hgb value below normal levels.4 Normal Hgb is 12 to 14 g/dL for females and 14 to 18 g/dL for males.5 These ranges differ, in part due to a loss of blood in menstruating females and decreased red blood cell production in males from androgen secretion.
Anemia in Patients with Cancer
Anemia can result from
insufficient production or destruction, rapid loss, or dysfunction of red
blood cells. In patients with cancer, anemia can have multiple etiologies.
Chemotherapy or radiation fields involving the marrow can suppress production
of erythropoietic stem cells. In addition, renal dysfunction and reduction in
erythropoietin release will impact the rate of erythropoiesis. Inadequate iron
storage can result in insufficient capacity of red blood cells to transport
oxygen. Iron deficiency can be the result of inadequate iron intake or blood
loss. This is a particular concern with patients who have cancer, since
treatment often suppresses the desire and ability to eat an adequate and
nutritious diet. Destruction (hemolysis) and acute blood loss can also
culminate with anemia.
Anemia may cause low Hgb in patients with cancer. Inflammation or neoplastic disease drives the release of cytokines, which can inhibit production of stem cells. Cytokines interferon-alpha, -beta, and -gamma, tumor necrosis factor (TNF)-alpha, and interleukin-1 may also induce apoptosis of red blood cells by macrophages.6 The bone marrow exhibits inadequate response to erythropoietin. Although high levels of iron are found in the marrow, an inadequacy exists in the transfer of iron to the red blood cells.
The European Cancer Anaemia Survey (ECAS), a study of 15,346 patients with cancer, revealed that 75% of patients who were treated with chemotherapy were anemic (Hgb <12 g/dL).7 In addition, Groopman et al. reviewed published chemotherapy trials to document the incidence of chemotherapy-induced anemia. In patients with non–small cell lung cancer who were treated with paclitaxel and a platinum agent (carboplatin or cisplatin), grade 3 (defined as Hgb of 6.5 to 7.9 g/dL) or 4 anemia (defined as Hgb <6.5 g/dL) was seen in 5% to 23% of patients. Among patients with breast cancer who had anthracycline-resistant disease and were receiving high-dose paclitaxel, 30% experienced grade 3 or 4 anemia.8,9
This review addresses only treatment-related anemia in patients with cancer; however, many of the points addressed can be applied to anemia of chronic disease.
Identifying Patients with Anemia
Individuals are usually
symptomatic when Hgb decreases to 7 g/dL.10 Patients may present
with skin and mucosal changes, such as pallor of the skin, palms, oral mucous
membranes, and nail beds. Severe deficiency can also lead to smooth tongue,
brittle nails, and cheilosis (scaling and fissuring of the lips).11
Additional symptoms can include weakness, fatigue, lethargy, dyspnea with
minimal exertion, chest pain, palpitations, and impaired concentration.
Patients may report bounding pulses and "roaring in the ears."
12
Patient assessment should include probing questions about the onset and severity of fatigue and cardiac symptoms. The patient's medical history (i.e., comorbidities, chemotherapy and radiation courses, medications) and laboratory blood analyses are important factors in determining the level and cause of anemia. Peripheral smears may be less frequently available for patients with cancer, but may prove useful when determining the etiology of anemia.
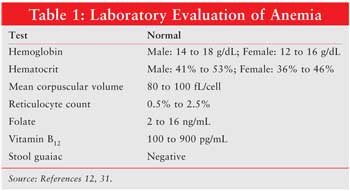
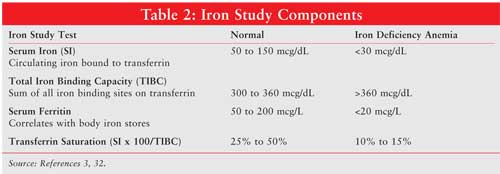
Laboratory tests that indicate anemia include Hgb, hematocrit, mean corpuscular volume (MCV), reticulocyte count, peripheral smear, folate, vitamin B12, stool guaiac, and iron studies (see Tables 1 and 2). Hematocrit reflects the volume of blood that is occupied by red blood cells. In a patient with iron-deficiency anemia, MCV will be below 80 fL; a healthy patient will have an MCV 80 to 100 fL. Reticulocytes normally range from 50,000 to 100,000, and they occupy 0.5% to 2.5% of blood volume. A peripheral smear can reveal visible red blood cell characteristics, such as color, size, shape, and content. Microcytic, or small blood cells (diameter <6 micrometers) can appear with iron deficiency anemia. Large, immature nucleated red blood cells, or macrocytic megoblasts (diameter >9 micrometers), can result from abnormal DNA replication. Cell division is halted while cytoplasmic maturation continues, producing a large immature red blood cell.3,13 Megoblastic anemia can also result from insufficient folate, another molecule essential for the production of DNA. In addition, vitamin B12 is necessary for DNA synthesis, and defects in intake or absorption of this vitamin can lead to megoblastic anemia. Stool guaiac tests for blood that has been excreted in the feces indicating abnormal bleeding. Iron studies include measurement of serum iron (circulating iron bound to transferrin), total iron binding capacity (TIBC), serum ferritin, and transferrin saturation (serum iron x 100/TIBC). Normal values for these laboratory tests and results indicating iron deficiency anemia are listed in Table 2. When inadequate iron is incorporated into the oxygen-carrying heme molecule, anemia can result.
Anemia can have a measurable effect on an individual's quality of life (QOL). The World Health Organization defines health as not only the absence of disease but also an individual's physical, mental, and social well-being.14 QOL is an acceptable measure of a patient's perception of their disease and treatment effects, as well as functional ability. The Functional Assessment of Cancer Therapy–General (FACT-G) is a validated 27-item questionnaire that is frequently used to measure QOL.15 Questions address the patient's physical, social, emotional, and functional well-being, and respondents rate answers on a scale of 0 to 4. Changes in two to three points on the physical and functional subscales of the FACT-G scales have been associated with meaningful changes in the level of patient activity.16 Subsets of the FACT include FACT-An (anemia) and FACT-F (fatigue).17 Fatigue can result from treatment-related effects (e.g., chemotherapy and radiation) as well as anemia. Studies have shown that fatigue has a longer duration in cancer patients following chemotherapy than do nausea, depression, or pain.18
Treating Anemia in Patients with Cancer
Erythropoietin therapy should be
considered when a cancer patient's Hgb reaches 10 to 11 g/dL.5
Symptom severity must also be taken into account, with immediate correction
(transfusion) considered when the Hgb falls below 8 g/dL.19 Prior
to the availability of erythropoietins, tranfusion was the only option for
severely anemic patients. However, transfusion is not without risks; blood is
of limited supply, and transfusion-related adverse events include infusion
reactions, volume overload, and lung injury.20
Recombinant erythropoietin stimulates red blood cell production in patients with anemia and fatigue caused by chemotherapy for cancer. The first of these agents was approved by the FDA in 1993. Erythropoietin is produced in the cells of the Chinese hamster ovary. Two products are licensed in the United States--epoetin alfa (Procrit, Epogen) and darbepoetin alfa (Aranesp). A third epoetin alfa product, Eprex, is available in Europe. Darbepoetin differs from the other recombinant erythropoietins due to its size; the molecule contains two additional oligosaccharide chains attached to sialic acid. Due to this unique feature, darbepoetin alfa has an extended half-life compared with epoetin alfa and beta.21
Both epoetin alfa and darbepoetin alfa are indicated for the treatment of anemia in patients with cancer who have nonmyeloid malignancies and anemia associated with chronic renal failure. Epoetin alfa carries additional indications for anemia in zidovudine-treated patients with HIV and reduction of transfusions in surgery patients. Contraindications for both products include uncontrolled hypertension (systolic blood pressures >140 mmHg or diastolic blood pressures >90 mmHg) and hypersensitivity to albumin.22 Pharmacists should emphasize the importance of remaining compliant with antihypertensive medications when counseling patients who are initiating therapy with erythropoietic agents. Epoetin alfa and darbepoetin alfa are typically formulated with albumin, although an albumin-free darbepoetin alfa solution is available.21,23
The initial dosage for epoetin alfa is either 150 units/kg IV or SC three times weekly or 40,000 units IV or SC weekly, whereas the initial dosage of darbepoetin alfa is 2.25 mcg/kg IV or SC weekly or 500 mcg IV or SC every three weeks. A darbepoetin alfa regimen that is commonly used in practice is 200 mcg IV or SC every other week. Numerous schedules have been tested for both products, including recent work exploring loading doses. Pateints prefer erythropoietin schedules that providing equivalent efficacy with fewer injections. Both epoetin alfa products and darbepoetin alfa require refrigeration and should be allowed to reach room temperature prior to SC injection. Shaking the products will denature the proteins and render them less effective.21,23
A number of studies have demonstrated the efficacy of epoetin alfa. In a randomized, controlled trial involving 344 patients with cancer who were on myelosuppressive chemotherapy, patients received either epoetin alfa 40,000 units SC weekly or placebo for 16 weeks. Patients on cisplatin-containing and noncisplatin-containing regimens were evenly distributed between the two study groups. Participants also received 325 mg of oral ferrous sulfate daily. Patients were anemic at the start of the study, with Hgb values of less than 11.5 g/dL for males and less than 10.5 g/dL for females. End points included increase in Hgb, percent of patients requiring transfusion, and improvement in QOL. If a participant's Hgb did not increase by 1 g/dL or a transfusion was given, the dosage was increased to 60,000 units a week. If the Hgb was 15 g/dL for two consecutive tests one week apart, treatment was discontinued. When Hgb fell to 13 g/dL, epoetin alfa was restarted at 75% of the previous dose. The incidence of adverse events was similar in the placebo and treatment groups (P >.05). Grade 3 or higher thrombotic vascular events occurred in five patients in the placebo arm (3%) and in eight (5%) patients in the epoetin alfa arm (P = NS). None of the cases of thrombosis were determined to be definitively related to the study drug. At study completion 72.7% of the epoetin alfa group had achieved at least a 2-g/dL increase in Hgb compared to 31.7% in the placebo group (P <.0001). The mean increase in Hgb was significantly greater for the treatment group (2.8 g/dL) compared to the placebo group (0.9 g/dL) (P <.0001). In the epoetin alfa arm, 25.3% received transfusions compared to 40% of those in the placebo arm (P = 0.005). QOL scores (measured using the FACT-An scale) increased by three points for patients treated with epoetin alpha but by only 0.6 points for patients treated with placebo (P = 0.18). Although the difference in QOL scores between the two groups was not statistically significant, the authors noted that patients with an increase in Hgb had improved QOL. In these patients with active cancers QOL is impacted by disease progression, pain, treatment regimen, and psychological state, as well as anemia. Correcting a single factor in a patient with cancer may have a small, yet important impact on QOL.24
Another study evaluating the impact of anemia-related fatigue on QOL in patients who were randomized to epoetin alfa (150 units SC three times weekly) or placebo for 12 to 24 weeks revealed that a significantly higher percentage of patients in the epoetin alfa group had increases in Hgb of 2 g/dL or greater compared to those in the placebo group (70.5% vs.19.1%, respectively; P <.001).25
Trials have been conducted measuring the effectiveness of darbepoetin alfa administered to cancer patients on chemotherapy. In a phase III, randomized, controlled trial with darbepoetin alfa, 320 patients with lung cancer who were treated with an expected 12-week course of a platinum-based regimen were randomized to darbepoetin alfa 2.25 mcg/kg/week or placebo for 12 weeks. Patients were included if they had Hgb ? 11.0 g/dL. Patients showing no response (Hgb increase less than 1 g/dL) at six weeks were titrated to an increased dosage of 4.5 mcg a week beginning at week 7. If a participant's Hgb exceeded 15 g/dL in males or 14 g/dL in females, dosing was withheld. Darbepoetin was restarted at 50% of the last dose when Hgb decreased to 13 g/dL or less. End points included percent hematopoietic response (patients achieving at least a 2-g/dL increase in Hgb or a Hgb level of 12 g/dL), percent requiring transfusion, and improvement in fatigue symptoms. Fatigue was assessed using the FACT-F scale. Hypertension was reported in 6% (nine patients) of the darbepoetin alfa group and 4% (six patients) of the placebo group. Thrombotic events occurred in 5% of the darbepoetin alfa group compared with 3% of the placebo group. Of the patients in the treatment group, 66% were responders, compared with 24% of patients in the placebo group (P <.001). Transfusions were required less frequently in the treatment group (27% vs. 52% for the darbepoetin alfa and placebo groups, respectively; P <.001). Once again, improvement in fatigue was not statistically significant, although patients in the darbepoetin alfa group reported a 56% improvement in the FACT-F scale, whereas patients in the placebo group reported a 44% improvement.26
Epoetin alfa was compared to darbepoetin alfa in a randomized, open-label trial of patients with breast cancer (n = 142), non–small cell lung cancer (n = 104), and cancer of the ovary, cervix, or uterus (n = 72). Patients on concurrent chemotherapy received darbepoetin alfa 200 mcg SC every two weeks or epoetin alfa 40,000 units SC weekly for up to 16 weeks. This trial was designed to validate a tool used to assess patient convenience when administering injectable anemia treatment using, the Patient Satisfaction Questionnaire for anemia (PSQ-An). If patients failed to achieve a Hgb increase of at least 1 g/dL after four weeks of treatment, the dosage was increased to 60,000 units SC weekly for epoetin alfa and 300 mcg every two weeks for darbepoetin alfa. For patients reaching or exceeding a Hgb value of 13 g/dL, the drug was withheld until Hgb decreased to less than 13 g/dL. Treatment was then restarted at the last dose received. Secondary end points included efficacy (i.e., the ability of both agents to achieve and maintain a Hgb level of 11 to 13 g/dL, transfusion requirements) and safety. The mean change in Hgb compared with baseline was similar in the darbepoetin alfa and epoetin alfa groups (82% and 86%, respectively), as was the incidence of transfusions (16% and 17%, respectively). Three treatment-related serious events were reported--one pulmonary embolism and one deep vein thrombosis in the epoetin alfa group and one deep vein thrombosis in the darbepoetin alfa group. Researchers concluded that darbepoetin alfa 200 mcg every two weeks and epoetin alfa 40,000 units weekly appear to achieve similar clinical and hematologic results.27
Trials of both epoetin alfa and darbepoetin alfa have demonstrated severe adverse effects, primarily in patients on dialysis or those with chronic renal failure. These adverse effects include hypertension, seizures, thrombotic events, and pure red cell aplasia.21,23 Pure red cell aplasia (PRCA) is a form of severe anemia characterized by a drop in Hgb of 1 g/dL/day, low reticulocyte count, reduction in bone marrow erythrocytes, and resistance to therapy with recombinant erythropoietin. Three cases were reported in patients (chronic kidney disease patients) with long-term use of erythropoetic agents from 1988 to 1998. The epoetin alfa product Eprex (not available in the U.S.) was reformulated in 1998 to replace albumin with polysorbate 80 and glycine. From 1998 to 2003, a total of 191 cases of PRCA were reported worldwide.28
In the Correction of Hemoglobin and Outcome in Renal Insufficiency (CHOIR) Trial, patients with chronic kidney disease were randomized to receive a dose of epoetin alfa targeted to achieve a Hgb of 13.5 g/dL (n = 715) or 11.3 g/dL (n = 717). The primary end point was a composite of death, myocardial infarction, stroke, and hospitalization for congestive heart failure and stroke. More events were seen in the group with a target Hgb of 13.5 g/dL (125 events) compared with the group with a target Hgb of 11.3 g/dL (97 events). Researchers concluded that in patients with chronic kidney disease, higher Hgb targets are associated with an increased risk of reaching the composite end point.29
Guidelines: Two guidelines are available to provide support for the use of erythropoietin for cancer patients. The 2006 update of the National Comprehensive Cancer Network (NCCN) guidelines advise considering erythropoietin therapy in patients with a Hgb value of 11 g/dL or less, while the American Society of Oncology and the American Society of Hematology (ASCO, ASH 2002) note a Hgb value of less than 10 g/dL as the starting point. Both NCCN and ASCO/ASH state that doses should be titrated to maintain a Hgb level of 12 g/dL.5,30
According to the NCCN guidelines, responders are defined as patients with an increase in Hgb of at least 1 g/dL within four weeks of initiating treatment with epoetin alpha and six weeks of initiating darbepoetin alfa. Patients should continue to receive erythropoietin until they reach a Hgb level of 12 g/dL. For those who do not respond, the dose should be titrated. If patients continue to be nonresponders after an additional four weeks at a higher dose of epoetin alfa or six weeks at a higher dose of darbepoetin alfa, erythropoetic agents should be stopped. For patients with rapidly escalating Hgb (an increase of >1 g/dL in two weeks), the dosage should be reduced by 25%.5
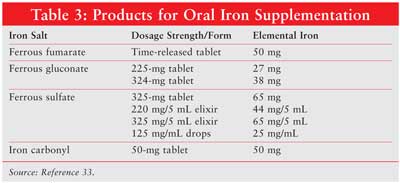
Iron Supplementation: Iron supplementation is frequently required for patients receiving erythropoietic agents. In ambulatory patients, oral products are used, with each formulation containing different amounts of elemental iron (Table 3 ). Ferrous fumarate, ferrous gluconate, and ferrous sulfate contain 33%, 12%, and 20% elemental iron, respectively. The percentage of absorbed iron decreases as the dose increases, and patients can experience constipation, dark stools, or nausea. A typical dosage is 50 to 100 mg elemental iron three times daily for six months. Caution should be employed in patients with hepatic disease, since the liver stores iron.
Conclusions
Consideration and treatment of
all possible causes of anemia are key to providing effective treatment and
achieving the goal of improved QOL. Following evaluation and treatment of
other etiologies of anemia in cancer patients with nonmyeloid malignancies who
are receiving chemotherapy, erythropoietic agents such as epoetin alfa or
darbepoetin alfa should be considered depending on the patient's
symptomatology when Hgb falls below 11 g/dL. If patients are responsive to
erythropoetic agents, therapy should be continued at the same dose until
achieving a Hgb of 12 g/dL. When Hgb reaches 12 g/dL or greater, the
erythropoetic agent should be discontinued. Following discontinuation, if Hgb
falls to 10 g/dL, therapy should be resumed. For nonresponders, the dosage
should be titrated. If no response is achieved after eight weeks for epoetin
alfa therapy or 12 weeks of darbepoetin alfa therapy, other causes of anemia
should be considered. Iron levels should be monitored monthly, and patients
should receive iron supplements as indicated.
References
1. Nathan D.
Hematologic Diseases. In: Cecil Textbook of Medicine. 20th ed.
Philadelphia: WB Sanders; 1996:817-841.
2. Martini, F.
Fundamentals of Anatomy & Physiology. 5th ed. Upper Saddle River, NJ:
Prentice Hall; 2001:628-633.
3. Hillman, R.
Hematopoietic agents: growth factors, minerals and vitamins. In: Hardman J,
Limbird L, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics
. 10th Ed. New York: McGraw-Hill; 2001:1487-1518.
4. Beers MH, Porter RS,
Jones TV, et al, eds. Merck Manual of Diagnosis and Therapy. 18th ed.
Whitehouse Station, NJ: Merck Research Laboratories; 2006.
5. National
Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology.
Cancer-and treatment-related anemia. V.2.2006. Accessed on November 20, 2006.
Available at www.nccn.org/professionals/physician_gls/PDF/anemia.pdf.
6. Weiss G, Goodnough
LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023.
7. Barrett-Lee PJ,
Ludwig H, Birgegard G, et al. Independent risk factors for anemia in cancer
patients receiving chemotherapy: results from the European Cancer Anaemia
Survey. Oncology. 2006;70:34-48.
8. National Cancer
Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
Accessed on December 1, 2006. Available at ctep.cancer.gov/reporting/ctc.html.
9. Groopman JE, Itri
LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl
Cancer Inst. 1999;91:1616-1634.
10. Hemphill R. In:
Tintinalli J, Stapszynski J, Kelen G, eds. Emergency Medicine. 6th ed.
New York: The McGraw-Hill Companies, Inc.; 2004:1319-1323.
11. Linker C. Blood.
In: Tierney L, McPhee S, Papadakis M, eds. Current Medical Diagnosis and
Treatment. 46th ed. New York: The McGraw-Hill Companies, Inc. 2006:493-547.
12. Approach to the
patient with anemia. UptoDate online 14.3. Accessed December 18, 2006.
Available at: www.uptodate.com.
13. Ryan D. Examination
of the blood. In: Lichtman M, Beutler E, et al. eds. Williams Hematology
. 7th edition. NewYork: The McGraw-Hill Companies, Inc.
14. World Health
Organization. Accessed December 13, 2006. Available at: www.who.int/about/en/
15. Cella DF, Tulsky
DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale:
development and validation of the general measure. J Clin Oncol.
1993;11:570-579.
16. Cella D, Hahn EA,
Dineen K. Meaningful change in cancer-specific quality of life scores:
differences between improvement and worsening. Qual Life Res.
2002;11:207-221.
17. Blohmer JU, Dunst
J, Harrison L, et al. Cancer-related anemia: biological findings, clinical
implications and impact on quality of life. Oncology. 2005;68(Suppl
1):12-21.
18. Curt GA, Breitbart
W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients:
new findings from the Fatigue Coalition. Oncologist. 2000;5:353-360.
19. Seidenfeld J, Piper
M, Flamm C, et al. Epoetin treatment of anemia associated with cancer therapy:
a systematic review and meta-analysis of controlled trials. J Natl
Cancer Inst. 2001;93:1204-1214.
20. Bohlius J, Weingart
O, Trelle S, Engert A. Cancer-related anemia and recombinant human
erythropoietin--an updated overview. Natl Clin Pract Oncol.
2006;3:152-164.
21. Amgen Inc. Aranesp
package insert. Thousand Oaks, CA. June 2006.
22. The seventh report
of the joint national committee on prevention, detection, evaluation and
treatment of high blood pressure. U.S. Department of Health and Human Services
National Institutes of Health. May 2003.
23. Amgen Inc. Procrit
package insert. Thousand Oaks, CA. November 2006.
24. Witzig TE,
Silberstein PT, Loprinzi CL, et al. Phase III, randomized, double-blind study
of epoetin alfa compared with placebo in anemic patients receiving
chemotherapy. J Clin Oncol. 2005;23:2606-2617.
25. Littlewood TJ,
Bajetta E, Nortier JW, et al. Effects of epoetin alpha on hematologic
parameters and quality of life in cancer patients receiving nonplatinum
chemotherapy: results of a randomized, double-blind, placebo-controlled trial.
J Clin Oncol. 2001;19:2865-2874.
26. Vansteenkiste J,
Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase
III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy.
J Natl Cancer Inst. 2002;94:1211-1220.
27. Schwartzberg LS,
Yee LK, Senecal FM, et al. A randomized comparison of every-2-week darbepoetin
alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia
in patients with breast, lung, or gynecological cancer. Oncologist.
2004;9:696-707.
28. Bennett C,
Cournoyer D, Carson KR, et al. Long-term outcome of individuals with pure red
cell aplasia and antierythropoietin antibodies in patients treated with
recombinant epoetin: a follow-up report from the Research on Adverse Drug
Events and Reports (RADAR) Project. Blood. 2005;106:3343-3347.
29. Singh AK, Szczech
L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney
disease. N Engl J Med. 2006;355:2085-2098.
30. Rizzo JD, Lichtin
AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based
clinical practice guidelines of the American Society of Clinical Oncology and
the American Society of Hematology. Blood. 2002;100:2303-2320.
31. Waddelow T, Sproat
T. Anemias. In: Dipiro J, Talbert R et al, eds. Pharmacotherapy: A
Pathophysiologic Approach. New York: Mcgraw-Hill; 2002:1731-1733.
32. Adamson J. Iron
deficiency and other hypoproliferative anemias. In: Kasper D, Braunwald E, et
al. eds. Harrisons Internal Medicine. 16th ed. NewYork: The McGraw-Hill
Companies, Inc.; 2005:586-592.
33. The University of
Texas M. D. Anderson hospital formulary, Houston TX. Accessed November 26,
2006. Available at
inside.mdanderson.org/departments/pharmacy/pdf/ironsupplements.pdf.
To comment on this article, contact editor@uspharmacist.com.





