US Pharm. 2007;32(1)(Oncology suppl):22-28.
ABSTRACT: Emesis as an adverse event of
antineoplastic therapy can be troublesome for many oncology patients.
Inadequately controlling emesis can have a major impact on patient care and
may even lead to refusal of treatment. Antiemetic treatment guidelines have
been established to help health care professionals better understand and
effectively treat this unpleasant side effect of chemotherapy. The American
Society of Clinical Oncology (ASCO) previously created antiemetic treatment
guidelines to assist clinicians in caring for oncology patients dealing with
emesis. Recently, ASCO's Update Committee revisited the prior guidelines, as
well as any new literature available on treating chemotherapy-induced emesis;
as a result, a more comprehensive, updated version is now available. This
article presents the conclusions and recommendations set forth by the ASCO
committee regarding the management of antiemetics in oncology patients.
In early 2006, the American Society
of Clinical Oncology (ASCO) set out to update their 1999 treatment guidelines
regarding antiemetics in oncology.1 An Update Committee was formed
to assess all published literature pertaining to the use of antiemetics in
oncology from 1998 to February 2006. Data reviewed by the committee included
systematic reviews and meta-analyses, as well as randomized controlled trials.
When the committee reviewed
and analyzed the literature results, they focused on the incidence of complete
response--when there are no episodes of emesis and no rescue medications are
dispensed after antineoplastic therapy is administered. Compared to nausea,
complete response is the recommended clinical outcome measure for development
of the guidelines, due to its objectivity. In contrast, nausea--the patient's
perception that emesis may occur--is more subjective.
The committee also reviewed
the treatment guidelines discussed at the Antiemetic Consensus Conference,
hosted by the Multinational Association of Supportive Care in Cancer (MASCC)
in 2004. This international conference facilitated consensus statements and
recommendations by representatives from nine oncology organizations, which
would be published in whole or in part with their individual organization's
antiemetic guidelines. This meeting took place in the hope that although
guidelines would inevitably differ between the associations, there would be a
smaller number of discrepancies between the individual documents on
antiemetics. The ASCO Update Committee used the findings from this meeting to
expand on their own recommendations and to aid in preparing their update
document.1
Impact and Risk Factors of
Chemotherapy-Related Emesis
Nausea and vomiting
are the two adverse effects involved with chemotherapy that tend to be the
most bothersome for patients and afflict the caregiver's or health care
professional's ability to appropriately treat a patient.2
Although nausea and vomiting seem common and devoid of significant harm, they
can cause dehydration, malnutrition, electrolyte imbalance, treatment delays
or refusal to continue treatment, and great discomfort.
Researchers have spent many
years trying to determine the physiological mechanism of emesis and have made
many strides toward effective therapies; however, the exact cause of emesis is
still not entirely understood. Today's studies on receptors found in the
chemoreceptor trigger zone (CTZ), e.g., dopamine, serotonin, and neurokinin,
have made great advances for patients prone to emesis during chemotherapy
treatment.2
There are several factors that
can raise the risk of nausea and vomiting, such as female gender, younger
patients (<50 years), those who have previously not improved with chemotherapy
regimens, and patients who do not have a history of chronic, high alcohol
intake and/or who experience significant fatigue.2 ,3
Non–patient-related factors that influence a patient's susceptibility to
emesis during treatment include the specific chemotherapy agent being used,
dose (especially high doses), schedule, and route in which the suspected agent
is given, as well as frequent-interval dosing and intravenous administration.
3 Nausea and vomiting can create additional havoc for patients already
coping with many other stresses. Consulting a health care professional about
risk factors and treatment options may reduce the risk of chemotherapy-related
emesis.
Types of
Chemotherapy-Induced Nausea and Vomiting
Patients can
experience nausea and vomiting minutes to hours after chemotherapy is
administered.1-3 This quick onset of emesis is considered acute
nausea and vomiting and usually ceases within the initial 24 hours after
therapy. When a patient experiences an episode that arises after the first day
following treatment, it can be classified as delayed nausea and vomiting
. Cisplatin is a prime example of emesis that can occur some time after
treatment. In some cases, cisplatin emesis may be at its most intolerable 48
to 72 hours after chemotherapy and can even extend up to seven days in some
cases. Anticipatory nausea and vomiting occurs when a patient has had a
previous negative experience with chemotherapy. This frequently happens in
patients who are preparing for their next treatment; they anticipate emesis
and consequently cause themselves to be sick. Breakthrough and
refractory nausea and vomiting are two other types of emesis that,
although uncommon, tend to arise in a certain subset of people. Breakthrough
vomiting occurs when the patient is undergoing current chemotherapy and is
receiving optimal antiemetic treatment but is still vomiting. Refractory
vomiting occurs after one or several chemotherapy cycles even though
antiemetic prophylaxis was used. The prophylaxis regimen is no longer
effective and the patient has stopped responding to the treatment.1-3
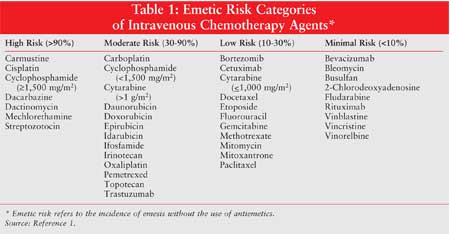
Emetic Risk Categories of Antineoplastic
Agents
While a few antineoplastic agents
have unquestionably confirmed their potential to cause emesis after
administration, most agents are classified according to risk based on
experience.1,4 Antineoplastic agents are grouped by the degree of
emetic risk they pose. The percentage of emetic risk is a key factor in
deciding which antiemetic treatment should be employed. ASCO has instituted
four emetic risk categories that were adopted from the recommendations created
during the MASCC conference of 2004: high, moderate, low, and minimal emetic
risk (Table 1).1,4
Medications Used to Treat
Chemotherapy-Related Emesis
Treatment for
chemotherapy-related emesis has remained the same, to an extent.1
Along with standard medications used to treat this adverse effect, an
additional agent has become available that was not yet approved when ASCO
published its guidelines in 1999. Since the approval of aprepitant, it has
proven through numerous studies to be a worthy addition to the antiemetic
treatment regimen for oncology patients.1,2
5-Hydroxytryptamine-3
(5-HT3) Serotonin Receptor Antagonists: This class of
medication, which includes dolasetron, granisetron, ondansetron, palonosetron,
and tropisetron, has been the primary treatment for nausea and vomiting for
many years. 5-HT3 serotonin receptor antagonists work by blocking
serotonin release to the CTZ, alleviating emesis in most patients. Debate
still looms over which 5-HT3 antagonist is the preferred treatment;
while some studies have shown noninferiority among the agents, there are still
lingering questions regarding a possible superior agent.1
Corticosteroids:
While both dexamethasone and methylprednisolone have been used to treat
chemotherapy-induced nausea and vomiting, dexamethasone is the most widely
available and commonly studied. The high therapeutic index of corticosteroids
and their efficacy as single agents in patients receiving chemotherapy of low
emetic risk establishes this class as the most frequently used antiemetics.
1
Neurokinin-1 (NK1
) Receptor Antagonists: The most recent agent included in the
antiemetic guidelines is the NK1 receptor antagonist known as
aprepitant.1 Aprepitant acts specifically on NK1
receptors in the brain and has little affinity for any other neurokinin
receptors. This NK1 receptor antagonist competitively inhibits
substance P from binding to NK1 receptors in the brain, thus
preventing emesis. Aprepitant is distinctive from other antiemetic agents in
regard to mechanism of action and its unique potential for CYP-related drug
interactions.1,2 Aprepitant serves as a substrate, as well as a
moderate inhibitor and inducer of cytochrome P-450 3A4 (CYP3A4) isoenzymes.
Aprepitant, acting as an inhibitor, can interact with corticosteroids (CYP3A4
substrates), causing the need for decreased dosing. In addition, several
chemotherapy agents (e.g., cyclophosphamide and docetaxel) are cleared by
CYP3A4, potentially inhibiting clearance of these drugs and increasing the
toxicity of some agents. There is no evidence of clinical complications when
aprepitant is given at its standard dose and schedule with chemotherapy.
1,2
Second-Line Agents:
Patients who are unable to tolerate or are refractory to first-line
antiemetics can use such agents as metoclopramide, butyrophenones,
phenothiazines, cannabinoids, benzodiazepines, and antihistamines. Lorazepam
and diphenhydramine can be used as adjuncts to first-line antiemetic regimens.
1
2006 Updated Guidelines
In June 2006, ASCO
released their most recent guidelines on the use of antiemetics in oncology.
Last updated in 1999,5 these guidelines have stayed the same in
various areas and have been modified or completely changed in others. All
treatment recommendations discussed refer to antineoplastic agents
administered intravenously.
High-Risk Guidelines (>90%)
Acute Emesis
(0 to 24 hours after chemotherapy): In terms of treating
nausea and vomiting in patients who experience symptoms due to chemotherapy of
high emetic risk (cisplatin and noncisplatin groups) within 24 hours after
treatment, three agents are included in the 2006 guidelines.1 5-HT
3 serotonin receptor antagonists, corticosteroids (dexamethasone), and
aprepitant are all effective in the acute treatment of emetic symptoms related
to chemotherapy. The addition of aprepitant to ASCO's updated guidelines is
the one major difference from their previous recommendations. The Update
Committee, after analyzing numerous studies involving these three agents and
cisplatin, states that all three agents improve the symptoms of
cisplatin-induced emesis.1 The committee is able to generalize this
claim to include other agents in the high-risk emesis category due to the
universal incidence of emesis with cisplatin (>99%). The incidence is so great
that if an antiemetic agent is effective against cisplatin, efficacy against
other chemotherapy agents in this high-risk category is assumed.1,6
Delayed Emesis
(?24 hours after chemotherapy):
Recommendations for the prevention of delayed emesis in oncology patients
receiving cisplatin and other agents of high emetic risk include the use of
dexa methasone and aprepitant.1 This two-drug combination for
the prevention of cisplatin and noncisplatin delayed emesis is a change from
previous guidelines, which advised the use of a corticosteroid alone or
in combination with metoclopramide or a 5-HT3 antagonist.1
The combination of a 5-HT3 serotonin receptor antagonist with
dexamethasone is no longer recommended by the Update Committee due to study
results suggesting inferiority of the combination compared to dexamethasone
alone.7-9 Furthermore, a trial published in 2005 found
dexamethasone in combination with aprepitant superior to dexamethasone in
combination with ondansetron, thus supporting ASCO's most updated
recommendation of dexamethasone and aprepitant.10
Moderate-Risk Guidelines
(30% to 90%)
Acute Emesis:
This updated category includes some agents previously considered to carry
high emetic risk in the original 1999 guidelines.1 Currently, the
Update Committee recommends a three-drug combination--a 5-HT3
serotonin receptor antagonist, dexamethasone, and aprepitant--for all patients
receiving anthracycline and cyclophosphamide (AC). For patients who receive an
antineoplastic agent of moderate emetic risk other than AC, ASCO recommends a
two-drug combination: a 5-HT3 serotonin receptor antagonist and
dexamethasone.1 While this combination for all moderate-risk agents
(excluding AC) has not changed since the previous guideline,
aprepitant was added to regimens involving AC due to recent studies indicating
increased emetic potential with these two antineoplastic agents, along with
trials showing the efficacy of aprepitant against these agents.11
It is important to note that in this particular case, regardless of
aprepitant's potential CYP3A4 inhibition, the Update Committee advises that
the concomitant use of cortico steroids should not be reduced with the
administration of aprepitant.1
Delayed Emesis:
For patients who receive AC therapy, single-agent aprepitant should be
used to prevent delayed-onset emesis; this is a change from the original
antiemetic guidelines. Dexamethasone as a single agent or a 5-HT3
serotonin receptor antagonist is preferred for delayed emesis regarding all
other agents of moderate emetic risk. 1
Low-Risk Guidelines (10% to
30%)
Acute Emesis: The 1999
guidelines recommended no antiemetic agent for the prevention and/or treatment
of chemotherapy-induced emesis. However, the updated recommendations advise 8
mg of dexamethasone for the treatment of patients at low emetic risk. Although
the majority of patients given low-risk chemotherapy do not experience emesis,
many patients do undergo this side effect. No relevant clinical trials address
an optimal agent to be used for antineoplastics of low risk.1
Delayed Emesis:
Preventive use of antiemetic agents for delayed emesis is discouraged in
patients who receive low-risk chemotherapy.1
Minimal-Risk Guidelines
(<10%)
Acute and
Delayed Emesis: This risk category is a new addition in the 2006
antiemetic guidelines; a number of agents in this category were considered low
risk in 1999.1 Bleomycin, 2-chlorodeoxyadenosine, fludarabine,
vinblastine, and vincristine are among the chemotherapy agents that are now
categorized as minimal risk. Few antiemetic studies were appropriate for
guideline development regarding this risk category, since the risk of emesis
was so low in these agents. Trials assessing antineoplastics in this risk
category have not been conducted. Thus, recommendations are unchanged from the
original guideline for "low-risk" agents.
Regarding acute emesis with
minimal-risk agents, the routine administration of antiemetics is not advised.
Depending on the patient and history of poor emetic control with these agents,
a small percentage of patients may require pretreatment with antiemetics. In
these rare cases, a one-time dose of dexamethasone 8 mg, phenothiazine, or
metoclopramide (as needed) is common. Routine antiemetics for the prevention
of delayed emesis are also not indicated for agents in this category.
Other Emetic Problems
Combination
Chemotherapy: Guidelines remain unchanged for the use of antiemetics
in patients who receive combination chemotherapy regimens. Antiemetic
treatment should be determined based on the chemotherapy agent that poses the
greatest emetic risk.1
Numerous Consecutive
Days of Chemotherapy: Antiemetics appropriate to the specific risk
category of the chemotherapeutic agent should be administered for each day of
chemotherapy. This remains identical to the previous 1999 guidelines.1
Anticipatory Emesis:
Anticipatory emesis or conditioned emesis prevention and treatment guidelines
have not changed from the original recommendations. For the prevention of
anticipatory emesis, antiemetic treatment should be administered with initial
chemotherapy and should be the most appropriate choice in terms of emetic risk
for that particular chemotherapy agent. For the treatment of anticipatory
emesis, systematic desensitization, along with behavioral therapy, appears
effective and is recommended by the Update Committee. Although no prospective
trials have confirmed the efficacy of alprazolam and lorazepam in these
patients, both are recommended as treatment options by many committee members.
1
Emesis in Pediatric
Patients: Guidelines previously suggested the use of a 5-HT3
antagonist plus a corticosteroid in children receiving chemotherapy of high
emetic risk.1 Recent guidelines have expanded this recommendation
and now advise that moderate-risk chemotherapy be given with these
antiemetics. Cancer research involving the use of antiemetics in the pediatric
population is limited, but one important factor to be aware of in this
population is the large interpatient variations in clearance and metabolism of
antineoplastics. Due to this interpatient variation, substandard dosing of
antiemetic agents is possible.1 Higher weight–based dosing is
imperative in order to provide reliable protection against emesis in this
population.12,13
High-Dose Chemotherapy:
High-dose chemotherapy guidelines have not changed from the original
recommendations. A 5-HT3 serotonin receptor antagonist in
combination with a corticosteroid is advised in any patient who is receiving
an antineoplastic in extremely high doses. During their review of the
literature, ASCO found no existing study evaluating antiemetic agents in
high-dose chemotherapy. The Update Committee therefore recommends
investigation of aprepitant as a potential addition to this course of therapy
based purely on the highly emetic nature of increased doses of
antineoplastics. Caution is advised in patients who receive aprepitant for
this indication due to aprepitant's moderate CYP3A4 inhibition, potentially
leading to increased toxic effects of antineoplastic agents.1
Continued Emesis
Regardless of Prophylaxis: Despite treatment with recommended
antiemetic prophylaxis, emesis can still persist in some patients. The Update
Committee makes no change from previous guidelines and instead suggests that
health care professionals consider four elements: patient factors, antiemetic
regimen, addition of lorazepam or alprazolam, and the possibility of adding
either high-dose intravenous metoclopramide or a dopamine antagonist to the
treatment regimen.1
Radiation-Induced Emesis
Emetic risk associated with radiotherapy can vary depending on the type of treatment. Radiation therapy of high emetic risk can be difficult to control and occurs in a small number of patients. While the 1999 guidelines defined only three radiotherapy-induced emesis risk groups, the newest update from ASCO incorporated one additional emetic risk group (minimal risk [<30%]), which is seen in Table 2. 1
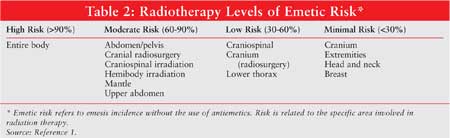
ASCO's current recommendations for
high radiation-induced emesis treatment are a 5-HT3 serotonin
receptor antagonist with or without a corticosteroid prior to each fraction
lasting at least 24 hours after treatment. Moderate- and low-risk
radiation-induced emesis recommendations involve administering a 5-HT3
serotonin receptor antagonist prior to each fraction. Lastly, in patients who
receive radiotherapy of minimal emetic risk, the Update Committee advises that
treatment be given on an as-needed basis with either dopamine or serotonin
receptor antagonists. The as-needed therapy should be continued
prophylactically for each additional radiation treatment day.1
Conclusion
Table 3
addresses preferred drug regimens for each emetic risk category. Dosage,
schedule, and route of administration for these agents can be found in the
2006 ASCO update.1 Table 4 summarizes the recommendations
for chemotherapy-induced emesis.

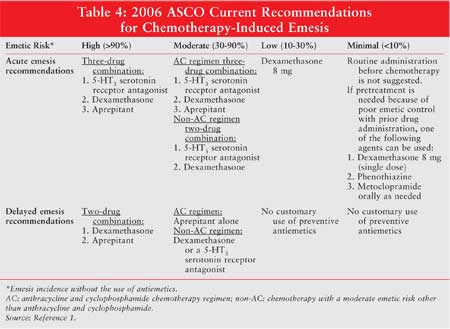
While these guidelines provide a strong foundation on which clinicians can base their treatment decisions, they should not be used as the definitive resource. Adherence to these guidelines is voluntary, and ASCO maintains that each patient's circumstance is different and the guidelines should be applied appropriately. Much of the literature and clinical trials that were reviewed by ASCO's Update Committee do not address specific treatment decisions that come up in practice. Thus, these updated guidelines are not only accessible for current recommendations but can also be used as a starting point for investigation in the future.
References
1. Kris MG, Hesketh PJ, et al. American Society of Clinical Oncology guideline
for antiemetics in oncology: update 2006. J Clin Oncol.
2006;24:2932-2947.
2. Dando TM, Perry CM. Aprepitant: A review of its use in the prevention of
chemotherapy-induced nausea and vomiting. Drugs. 2004;64:777-794.
3. American Cancer Society. Nausea and Vomiting: Treatment Guidelines for
Patients with Cancer. Atlanta: American Cancer Society, 2005. Available at:
www.cancer.org/docroot/CRI/content/CRI_2_4_4x_NCCN_Nausea_and_
Vomiting_Treatment_Guidelines_for_Patients_with_Cancer.asp. Accessed October
2, 2006.
4. Grunberg SM, Osoba D, et al. Evaluation of new antiemetic agents and
definition of antineoplastic agent emetogenicity--an update. Support
Care Cancer. 2005;13:80-84.
5. Gralla RJ, Osoba D, et al. Recommendations for the use of antiemetics:
evidence-based, clinical practice guidelines. J Clin Oncol.
1999;17:2971-2994.
6. Hainsworth JD. The use of ondansetron in patients receiving multiple-day
cisplatin regimens. Semin Oncol. 1992;19:48-52.
7. Latreille J, Pater J, et al. Use of dexamethasone and granisetron in the
control of delayed emesis for patients who receive highly emetogenic
chemotherapy. National Cancer Institute of Canada Clinical Trials Group. J
Clin Oncol. 1998;16:1174-1178.
8. Roila F. Prevention of cisplatin-induced delayed emesis: still
unsatisfactory. Support Care Cancer. 2000;8:229-232.
9. Gridelli C, Ianniello GP, et al. A multicentre, double-blind, randomized
trial comparing ondansetron versus ondansetron plus dexamethasone in the
prophylaxis of cisplatin-induced delayed emesis. Int J Oncol.
1997;10:395-400.
10. Aapro MS, Schmoll HJ, et al. Comparison of aprepitant combination regimen
with 4-day ondansetron + 4-day dexamethasone for prevention of acute and
delayed nausea/vomiting after cisplatin chemotherapy. J Clin Oncol.
2005;23:730s. Abstract.
11. Warr DG, Hesketh PJ, et al. Efficacy and tolerability of aprepitant for
the prevention of chemotherapy-induced nausea and vomiting in patients with
breast cancer after moderately emetogenic chemotherapy. J Clin Oncol.
2005;23:2822-2830.
12. Allen JC, Gralla R, et al. Metoclopramide: dose-related toxicity and
preliminary antiemetic studies in children receiving cancer chemotherapy. J
Clin Oncol. 1985;3:1136-1141.
13. Tsuchida Y, Hayashi Y, et al. Effects of granisetron in children
undergoing high-dose chemotherapy: a multi-institutional, cross-over study.
Int J Oncol. 1999;14:673-679.
To comment on this article, contact
editor@uspharmacist.com.





