US Pharm.
2008;33(7)(Oncology suppl):23-26.
ABSTRACT:
Cancer, chemotherapy, and surgery are conditions that increase the risk of the
development of venous thromboembolism (VTE), a common and potentially
life-threatening condition. The estimated incidence of VTE in cancer patients
is 1 in 200. Unfractionated heparin, low-molecular-weight heparin (LMWH), and
oral vitamin K antagonists are therapies used to treat VTE. Based on findings
of three key trials, LMWH therapy is recommended by several prominent
organizations for the management or prevention of VTE in patients with cancer.
Venous thromboembolism (VTE)
is a common condition that can manifest in many ways. It is often observed
clinically as either deep venous thrombosis (DVT) of the lower extremities or
pulmonary embolism (PE), which can be life-threatening. It is difficult to
determine the incidence of VTE since more than 50% of patients present with
clinically silent pathology.1 It has been suggested that VTE occurs
at a rate of 7.1 per 10,000 persons per year among community residents and 17
per 100,000 persons per year in the general population.2,3 VTE may
occur more frequently in men, with a slightly higher prevalence in African
American males.2 The incidence of VTE increases with age and almost
doubles with each decade of life beyond 50 years.1,2 Pathologic
factors associated with increased risk of VTE include circulatory stasis,
increased blood coagulability, and vessel-wall injury, commonly known as
Virchow's triangle or triad.1 The classic model of
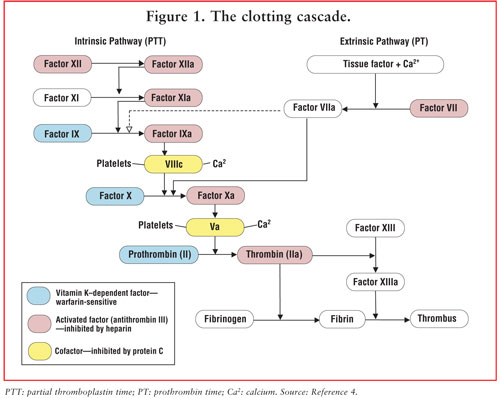
blood coagulation involves a cascade of precursor protein (zymogen) activation
reactions (FIGURE 1).4 At each stage, a zymogen is converted
to an active protease (denoted in FIGURE 1 by a lowercase "a") by
cleavage of one or more peptide bonds in the precursor molecule. The end
result of this cascade is the formation of an insoluble fibrin clot, or
thrombus.4
The risk of developing VTE
increases in the presence of cancer, chemotherapy, and surgery.2
Malignancy and chemotherapy augment the risk of VTE by greater than fourfold
and 6.5-fold, respectively, for an estimated yearly incidence of 1 per 200
patients with cancer.3 Cancer cells interact with monocytes and
macrophages releasing tumor necrosis factor, interleukin 1, and interleukin 6,
resulting in endothelial damage and creating a thrombogenic surface. The
interaction between tumor cells and macrophages also activates platelets,
factor XII- and factor X–generating thrombin, and ultimately, the potential
for thrombosis.5 Tumor type, stage or severity of disease, and
concomitant and adjunctive treatments all affect the risk of VTE in patients
with cancer.3
A recent study of patients
with lung cancer suggests that venous thrombosis may be more prevalent in
patients with adenocarcinoma (ACA) than in those with squamous cell carcinoma.
6 The interaction of carcinoma mucins with leukocyte L-selectin and
platelet P-selectin without thrombin generation is a reasonable molecular
explanation for the increased rate of venous thrombosis in patients with ACA.
6 Adenocarcinoma develops in cells lining glandular internal organs
such as the lungs, breast, colon, prostate, stomach, pancreas, and cervix.
7 Cancers more highly associated with VTE are those of the brain,
pancreas, ovary, and breast.3
Treatments for VTE include
unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and oral
vitamin K antagonists (VKAs) like warfarin. UFH and LMWH are indicated for
acute treatment of DVT; UFH, LMWH, and oral VKAs are indicated for chronic
treatment. Oral VKAs are initiated with UFH or LMWH and continued until two
international normalized ratios (INRs) 24 or more hours apart are within
appropriate range.3
The literature suggests that
LMWH may be superior to oral VKAs with respect to risk of recurrent VTE,
bleeding, and mortality.3 UFH and LMWH potentiate antithrombin and
block P-selectin and L-selectin, decreasing platelet aggregation.6
Both agents potentiate the activity of antithrombin III, which inhibits
activated coagulation factors X and II.8 UFH, unlike LMWH, requires
frequent monitoring due to its unpredictable dose response and narrow
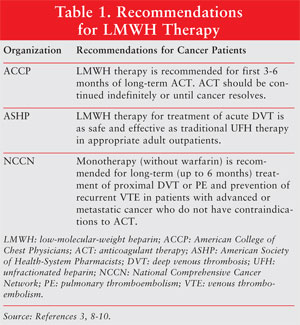
therapeutic window.3 Several prominent organizations support the
use of subcutaneous (SC) LMWH for three to six months, among them the American
College of Chest Physicians, the American Society of Health-System
Pharmacists, and the National Comprehensive Cancer Network.3 See
TABLE 1 for specific recommendations.3,8-10
Alternatives to standard oral
VKA therapy are under investigation for several reasons, namely the drug–drug
and drug–disease interactions and monitoring requirements associated with
warfarin.3 Most cancer treatments and adjunctive therapies are
complex, involving not only chemotherapy but also antiemetics, growth factors,
antibiotics, steroids, and pain medications. Cancer-related gastrointestinal
(GI) problems, malnutrition, and liver impairment may complicate
anticoagulation of patients with warfarin. In addition, chemotherapy-induced
thrombocytopenia and the need for invasive procedures may result in
subtherapeutic INRs, which may be perpetuated by warfarin's exceptionally long
half-life. The difficulty of maintaining a therapeutic INR (and perhaps the
inability to do so) may result in recurrence of VTE as well as potential
bleeding complications. Also, the frequent, close monitoring requirements for
warfarin may further erode quality of life in patients with cancer.
Several landmark trials have
been conducted that are the foundation for the aforementioned recommendations,
including CLOT, FAMOUS, and ONCENOX.
CLOT Study
CLOT (Low Molecular
Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous
Thromboembolism in Patients with Cancer) was the first large-scale study
comparing an oral anticoagulant with extended LMWH treatment in cancer
patients with VTE. CLOT was a multicenter, international, open-label,
clinical trial evaluating 676 patients with cancer and symptomatic proximal
DVT, PE, or both. Patients were randomized to receive an oral
anticoagulant or SC dalteparin (Fragmin). The first group (n = 338) received
SC dalteparin 200 IU/kg qd for five to seven days (maximum 18,000 IU),
followed by oral anticoagulant therapy (warfarin or acenocoumarol; target INR
2.5, range 2.0–3.0) for six months. The second group (n = 338) received
dalteparin alone for six months (200 IU/kg qd for one month, then
approximately 150 IU/kg qd for five months). Ninety percent of patients had
solid tumors and 67% had metastatic disease. Endpoints of the study included
incidence of symptomatic, recurrent DVT, PE, or both during the six-month
study period (primary) and events of major bleeding, any bleeding, and death
at six to 12 months (secondary).
During the six-month period,
the incidence of recurrent VTE in patients treated with dalteparin alone was
8% versus approximately 16% in the oral anticoagulant group (hazard ratio [HR]
0.48; P =.002). Out of 53 thrombotic events, 20 occurred in the oral
anticoagulant group when INR was below 2.0. Patients were above therapeutic
range 24%, below therapeutic range 30%, and at therapeutic range 46% of the
treatment time. The Kaplan–Meier estimate of risk of recurrent VTE at six
months was 9% in the dalteparin group versus 17% in the oral anticoagulant
group.11
There were no significant
differences between the dalteparin and oral anticoagulant arms, respectively,
in rates of major bleeding (6% vs 4%; P =.27), any bleeding (14% vs
19%; P =.09), or overall mortality (39% vs 41%; P =.53) at six
months. In each group, cancer progression was the cause of mortality 90% of
the time. The researchers concluded that the risk of recurrent DVT was
significantly lower with dalteparin compared with oral anticoagulant in VTE
patients with cancer. Some of the study's limitations were the open-label
design, wide variations in INRs in the oral anticoagulant group, and possible
bias in VTE symptom detection.11
A post hoc analysis of the
CLOT study results was performed in 602 of the patients with solid tumors to
determine whether a treatment-related difference in mortality existed
between patients with metastatic (n = 452) and nonmetastatic (n = 150) disease
at randomization. During 12-month follow-up, 70% of patients with metastatic
disease had died, with no difference in mortality between the two treatment
groups (HR 1.1; P =.46). In patients with nonmetastatic disease,
however, 12-month cumulative mortality was 20% for those in the dalteparin
group versus 35% in the oral anticoagulant group (HR 0.50; P
=.03). The results of the post hoc analysis are consistent with the theory
that LMWHs may exert clinically relevant antineoplastic effects in
nonmetastatic cancer.12
FAMOUS
FAMOUS (Fragmin
Advanced Malignancy Outcome Study) was a randomized, double-blind,
placebo-controlled, multicenter trial investigating the efficacy and safety of
chronic LMWH administration in cancer patients without a known history of
thrombosis. The primary endpoint of the study was mortality after one year of
therapy. Secondary endpoints were rates of symptomatic, objectively confirmed
VTE and bleeding complications. Inclusion criteria were age range 18 to 80
years and histologically confirmed advanced stage III or IV cancer of the
breast, lung, GI tract, pancreas, liver, genitourinary tract, ovary, or uterus
with an expected survival of at least three months. Patients were excluded if
they had an active bleeding disorder, known hypersensitivity to heparin, or a
platelet count less than 50,000/µL. Patients received either 5,000 IU
dalteparin qd or 0.9% normal saline qd.13
Survival estimates for the
dalteparin and placebo groups at one year were 46% (95% CI, 39%–53%) and 41%
(95% CI, 34%–49%), respectively (P =.19). Possible explanations
for the lack of statistical difference in survival between groups are that the
study was underpowered and that most patients had a relatively short life span
from baseline. In addition, these patients had highly advanced disease with
aggressive pathology. Though not a primary endpoint of this study, it is worth
noting that the survival rate at two years was 27% (95% CI, 20%–34%) in the
dalteparin group versus 18% (95% CI, 11%–25%) in the placebo group and the
rate at three years was 21% (95% CI, 14%–28%) versus 12% (95% CI, 5%–19%).
13
The study did successfully
establish low rates of symptomatic VTE and bleeding. Dalteparin was given
without routine monitoring of anti-Xa activity or platelet levels, suggesting
that dalteparin therapy at this dose is safe in this patient population. Thus,
this trial demonstrated the feasibility and safety of long-term dalteparin
administration in patients with advanced cancer. A post hoc analysis suggested
that dalteparin also might modify a tumor's angiogenic response and
physiologic behavior through gene upregulation; this possibility requires
further investigation.13
ONCENOX Study
ONCENOX study was a
randomized, open-label, multicenter trial that assessed the effectiveness and
safety of enoxaparin for prevention of recurrent DVT in cancer patients and
examined patient compliance. All patients initially received enoxaparin 1.0
mg/kg twice daily for five days and then received one of the following
regimens: enoxaparin 1.0 mg/kg qd (group 1), enoxaparin 1.5 mg/kg qd (group
2), or oral VKA therapy (group 3). The incidence of recurrent VTE was 7.1% in
group 1, 3.2% in group 2, and 10.3% in group 3. No differences in major or
minor bleeding rates were detected. Compliance rates were 97.6%, 94.1%, and
92.8% in groups 1, 2, and 3, respectively.14
Additional Considerations
The management of
cancer and thrombosis and the prevention of thrombosis in cancer patients are
complex processes that require due diligence in treatment considerations in
order to minimize treatment complications, preserve quality of life, and
reduce mortality risk. As with all treatment plans, efficacy, convenience,
compliance, and satisfaction must be considered. The type and location of the
cancer, comorbidities, concomitant therapies, and prognosis also must be
considered.
Key organizations and
associations, as previously discussed, support the use of LMWH therapy in
patients with cancer. LMWH therapy offers patients efficacy without the
intensive monitoring and dietary restrictions required with oral VKA therapy.
The risks and dangers associated with multiple drug–drug and drug–disease
interactions that often result in suboptimal anticoagulation with oral VKA
therapy are minimized significantly with LMWH. Compared with UFH, less
anticoagulant monitoring and shorter hospital stays are considerable benefits
of LMWH therapy.3
REFERENCES
1. Haines ST,
Zeolla M, Witt DM. Venous thromboembolism. In: DiPiro JT, Talbert RL, Yee GC,
et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New
York, NY: McGraw-Hill Medical; 2005:373-413.
2. Snow V, Qaseem A,
Barry P, et al. Management of venous thromboembolism: a clinical practice
guideline from the American College of Physicians and the American Academy of
Family Physicians. Ann Intern Med.2007;146:204-210.
3. Nishioka J, Goodin
S. Low-molecular-weight heparin in cancer-associated thrombosis: treatment,
secondary prevention, and survival. J Oncol Pharm Pract. 2007;13:85-97.
4. Beckett B,
Kwiatkowski JL. Coagulation disorders. In: DiPiro JT, Talbert RL, Yee GC,
Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic
Approach. 5th ed. New York, NY: McGraw-Hill Medical; 2002:1747-1750.
5. Castelli R, Porro F,
Tarsia P. The heparins and cancer: review of clinical trials and biological
properties. Vasc Med. 2004;9:205-213.
6. Blom JW, Osanta S,
Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients:
higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb
Haemost. 2004;2:1760-1765.
7. Anderson KN,
Anderson L, Glanze WD, eds. Mosby's Medical, Nursing and Allied Health
Dictionary. 4th ed. St Louis: Mosby-Year Book, Inc; 1994.
8. Büller HR, Agnelli
G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease:
the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.
Chest. 2004;126 (suppl 3):401S-428S.
9. ASHP therapeutic
position statement on the use of low-molecular-weight heparins for adult
outpatient treatment of acute deep-vein thrombosis. Am J Health Syst Pharm.
2004;61:1950-1955.
10. National
Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology.
Venous thromboembolic disease. V.1.2008.
www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed March 26, 2008.
11. Lee AY, Levine MN,
Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the
prevention of recurrent venous thromboembolism in patients with cancer. N
Engl J Med. 2003;349:146-153.
12. Lee AY, Rickles FR,
Julian JA, et al. Randomized comparison of low molecular weight heparin and
coumarin derivatives on the survival of patients with cancer and venous
thromboembolism. J Clin Oncol. 2005;23:2123-2129.
13. Kakkar AK, Levine
MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin,
and survival in advanced cancer: the fragmin advanced malignancy outcome study
(FAMOUS). J Clin Oncol. 2004;22:1944-1948.
14. Deitcher SR,
Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic
events (VTE) in patients with active malignancy: a randomized study of
enoxaparin sodium alone versus initial enoxaparin sodium followed by warfarin
for a 180-day period. Proc Am Soc Clin Oncol. 2003;22. Abstract 3060.
To comment on this article,
contact rdavidson@jobson.com.






