US Pharm. 2016;41(2):HS6-HS10.
ABSTRACT: Brain tumors are among the most complex and dynamic disease states many pharmacists will encounter. The intricacies and subtleties of treatment can be extremely difficult to navigate and explore. In general, risk factors are poorly understood, with little supporting evidence demonstrated for most purported factors. The presentation of symptoms can vary from patient to patient, with individual tumor types often dictating patient experience. The classification and treatment of gliomas depends largely on individual tumor presentation. Low-grade gliomas resemble their tissue of origin and often respond to resection, radiotherapy, and/or chemotherapy; high-grade gliomas and disease recurrence often must be treated more aggressively.
Gliomas are central nervous system (CNS) tumors that arise from glial cells in the brain or spine. Although brain tumors are a relatively rare disease, gliomas are the most common type, accounting for 81% of diagnosed malignant tumors.1 Gliomas are typically divided into four categories: astrocytomas, oligodendrogliomas, ependymomas, and mixed gliomas. Astrocytomas originate from astrocytes in the cerebellum and are typically reported to be the most common glioma, whereas oligodendrogliomas originate from oligodendrocytes in the CNS. Ependymomas, which are tumors arising from the ependyma, are relatively rare and are often differentiated from astrocytomas and oligodendrogliomas. Mixed gliomas, sometimes referred to as oligoastrocytomas, are malignant gliomas that contain multiple types of glial cells.2
Gliomas can be further categorized as low-grade or high-grade, which is an important consideration in prognosis and treatment assessment. Low-grade gliomas (LGGs) include tumors categorized grade I and grade II by the World Health Organization. High-grade gliomas (HGGs) include grade III and grade IV tumors. In general, LGGs look much more like their origin tissue and grow more slowly than HGGs, which can be highly differentiated and grow very quickly.3 With time to grow and develop, LGGs can progress into HGGs.4 Prognosis is usually much better in patients presenting with LGGs, as the slower-growing tumor is more easily resected and potentially treated with radiation and chemotherapy.5
Risk Factors
In general, risk factors for brain tumors are poorly understood and many purported theories are largely anecdotal. Factors such as carcinogens (e.g., tobacco smoke), head trauma, high-protein diets, and cell-phone use have been examined as potential causative factors in brain tumor development.6-8 Evidence for most of these proposals is lacking, and such causative links will remain a focus in future studies for better understanding of the disease state. Of the potential environmental causes examined, only ionizing radiation exposure seems to lead to an increase in brain tumor development.8,9 Brain tumor development has been seen in patients with past exposure to radiotherapy (RT), as well as in survivors exposed to radiation from atomic detonations in Hiroshima, Japan. Lesser exposure from x-rays and other diagnostic procedures probably poses little or no health risk.8 It has been suggested that viral infections, such as cytomegalovirus, have a possible association with brain tumors, but a link has yet to be proven.10 Genetics may also be a factor, since certain familial predisposition syndromes have been identified as having a potential association with brain tumors.6,7 More evidence is needed for a true understanding of individual risk factors.
Clinical Presentation
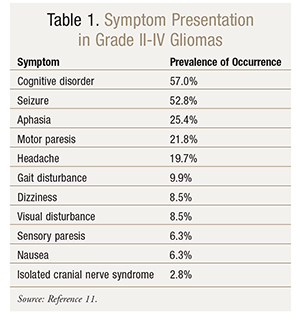
Like other types of brain tumors, gliomas can have an extremely fluid presentation (TABLE 1). Factors such as tumor size, location, and growth rate typically dictate general disease presentation. Small tumors may be asymptomatic for a long time, whereas large tumors may have a dramatic clinical presentation. Some symptoms that present with gliomas are caused by increased intracranial pressure; others are focal neurologic symptoms associated with the tumor itself. Common general symptoms in glioma patients include headache, gastrointestinal upset (including nausea and vomiting), cognitive dysfunction, and changes in personality.11,12 Brain tumor–associated headaches historically have been believed to be most severe early in the morning; however, later data suggest that this feature may be relatively uncommon.13 Seizures are frequently seen in brain tumor patients, especially those with low-grade gliomas.14 Specific manifestations of brain tumor–related seizures often depend on the location and type of tumor. Despite the relative frequency of seizure manifestations, the National Comprehensive Cancer Network does not recommend initiating seizure prophylaxis in brain tumor patients who have never had a seizure.5
Chemotherapy
General Considerations: Chemotherapy is often used in combination with surgery and radiation to treat brain tumors. Since chemotherapy for brain tumors targets the brain, particular attention must be paid to ensuring proper therapeutic drug concentration at the site of action. The brain is protected by the blood-brain barrier, a complex system separating circulating blood from the brain. The blood-brain barrier has specific permeability, making it difficult for many drugs to attain therapeutic concentration within the brain.15 Because of this, the dosage form and specific chemotherapeutic agents must be carefully considered in the treatment of brain tumors. Additionally, multiple factors, including patient demographics, tumor location and grade, and treatment options, must be closely examined. It is crucially important for an expert in the field of oncology to be involved in evaluating patients and selecting the optimal treatment.
LGG: There is no consensus regarding recommendations for initiation of treatment in LGGs. Originally, a diagnosis of LGG in young, healthy patients meant adopting a wait-and-see approach. This was due largely to the extremely unpredictable and potentially slow-growing nature of these tumors and was further validated in the 1990s by findings that there was no difference in outcomes when treatment was deferred. This approach fell out of favor after more recent data demonstrated that even mild LGGs often become malignant. Resection, RT, and chemotherapy are the current standard for treatment of LGGs. However, the determination should be made on a case-by-case basis owing to the high variability and complex nature of each patient and tumor.
Several chemotherapy options exist for the treatment of LGGs (TABLE 2). Temozolomide, a potent alkylating agent, is effective against glioblastomas and astrocytomas. Temozolomide is a prodrug converted in vivo to a cytotoxic alkylating agent metabolite; this metabolite then inhibits DNA replication and causes cell death.16 Often used in LGG therapy, temozolomide has been shown to be efficacious and may delay disease progression.17-19 Importantly, adjuvant temozolomide therapy should be continued after RT is completed. Hematologic toxicities are an expected adverse effect, so careful monitoring of blood counts is recommended.16 Opportunistic infections may also occur, so prophylaxis against Pneumocystis carinii pneumonia should be considered in patients receiving concurrent RT and temozolomide.5,16,17 Temozolomide appears to be well tolerated compared with other available regimens, which is important when chemotherapy is being considered.20,21 Glioma patients with known methylated O6-methylguanine DNA methyltransferase (MGMT) promoter methylation may exhibit a better treatment response than non-MGMT patients.5 Although temozolomide is recommended as a therapeutic option, it has not been well-studied against other agents. Head-to-head trials evaluating the efficacy and outcomes of temozolomide against other potential regimens and plans of action are necessary to determine which treatment demonstrates maximum benefit.
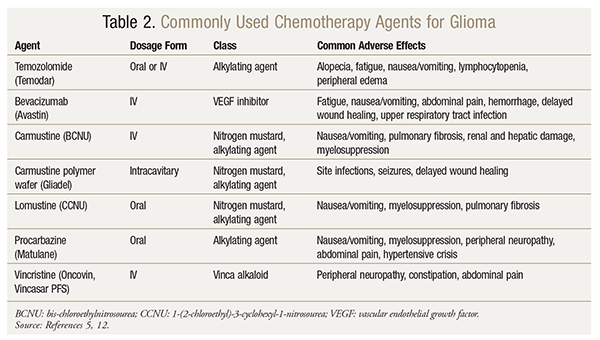
Another chemotherapy regimen used in LGG consists of procarbazine, lomustine, and vincristine (PCV). PCV also benefits median survival and decreases tumor size.18,22 Like temozolomide, procarbazine and lomustine are alkylating agents that cross the blood-brain barrier and affect tumor growth directly. Conversely, vincristine is a plant alkaloid that inhibits mitosis. Interestingly, vincristine does not cross the blood-brain barrier, leading some researchers to question its place in treatment.23 However, there is no evidence that excluding vincristine from the PCV regimen leads to comparable outcomes. Although there is more evidence demonstrating the advantages of the PCV regimen, temozolomide is preferred by some clinicians, as it is better tolerated and easier to administer.21,24 More research is needed to properly compare these two chemotherapy regimens.
Additionally, some data indicate that the use of platinum agents and other nitrosoureas have modest efficacy in some LGGs and can be considered an option for chemotherapy.25
HGG: As with LGG treatment, resection, RT, and chemotherapy are options to consider for HGG therapy. Treatment of newly diagnosed HGG is often similar to that for LGG in that temozolomide with postoperative RT is frequently employed, particularly for newly diagnosed HGGs in young patients, as well as for rechallenging in patients who previously received treatment.5,17,26 Temozolomide is also continued as adjuvant therapy after RT is completed.
Another option for the postoperative treatment of HGG is the application of implanted carmustine wafers.27 Implanted after tumor resection, this novel delivery method for carmustine bypasses the blood-brain barrier and delivers the drug slowly and directly to the site of action. This process allows minimal systemic absorption and adverse systemic effects, and it circumvents any inherent limitations presented by the blood-brain barrier.28 Although initially there were concerns about patients with implanted carmustine wafers receiving additional temozolomide therapy, evidence suggests that it is safe for patients to receive both therapies, but consideration of potential harm should be assessed.29
In most patients, HGG will eventually recur. Chemotherapy is less effective against recurrent HGG. There are many chemotherapeutic options, including further treatment with temozolomide, nitrosoureas, PCV, cyclophosphamide, or platinum-based regimens. Irinotecan and etoposide also may be considered.5,30
Bevacizumab is approved for the treatment of recurrent HGG. This vascular endothelial growth factor (VEGF) inhibitor inhibits the abnormal angiogenesis commonly present in recurrent glioma, suppressing further tissue growth and normalizing tumor vasculature.31 It is also hypothesized to increase the efficacy of other cytotoxic agents used to treat brain tumors. Bevacizumab may be used in combination with irinotecan, carmustine or lomustine, carboplatin, or temozolomide. Caution must be exercised, however, as adverse effects can be relatively serious and require careful monitoring.5 Patients may experience hypertension, delayed wound healing, and thromboembolism.31
Pharmacist’s Role
The pharmacist should assist in the dosing of individual chemotherapy agents, as well as in managing side effects related to treatment. Opportunity also exists for the pharmacist to monitor laboratory data for potential toxicities and to help develop and maintain chemotherapy protocols to further enhance patient safety. Each patient experiences chemotherapy differently, both emotionally and physically, so the pharmacist should counsel patients and provide coping techniques. Furthermore, the pharmacist can make important recommendations for the treatment of certain complications secondary to oncologic disease, including seizures, headache, nausea, and dizziness.
Conclusion
Cancers of the brain are extremely complicated and challenging to treat. This is attributable to a variety of factors, including the complexity of the disease and the fluid nature of available evidence-based literature. Pharmacists must be vigilant in order to stay informed on new treatments for and management of gliomas of all types. Careful attention must be paid to treatment, as adverse effects of appropriate chemotherapeutic agents can require an acute understanding necessary for maximum patient care. By maintaining an awareness of new and important information regarding the care and treatment of patients with gliomas, pharmacists can provide a valuable service to both patients and clinicians.
REFERENCES
1. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896-913.
2. Australian Cancer Network Adult Brain Tumor Guidelines Working Party. Clinical practice guidelines for the management of adult gliomas: astrocytomas and oligodendrogliomas. www.cancer.org.au/content/pdf/HealthProfessionals/ClinicalGuidelines/Clinical_Practice_Guidelines-Adult_Gliomas-AUG09.pdf. Accessed December 15, 2015.
3. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
4. Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-3140.
5. National Comprehensive Cancer Network. Central nervous system cancers. Version 1.2015. www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed December 16, 2015.
6. Braganza MZ, Rajaraman P, Park Y, et al. Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP Diet and Health Study. Br J Cancer. 2014;110:242-248.
7. Little MP, Rajaraman P, Curtis RE, et al. Mobile phone use and glioma risk: comparison of epidemiological study results with incidence trends in the United States. BMJ. 2012;344:e1147.
8. Idowu OE, Idowu MA. Environmental causes of childhood brain tumours. Afr Health Sci. 2008;8:1-4.
9. Pollak L, Walach N, Gur R, Schiffer J. Meningiomas after radiotherapy for tinea capitis—still no history. Tumori. 1998;84:65-68.
10. Söderberg-Nauclér C, Johnsen JI. Cytomegalovirus infection in brain tumors: a potential new target for therapy? Oncoimmunology. 2012;1:739-740.
11. Posti JP, Bori M, Kauko T, et al. Presenting symptoms of glioma in adults. Acta Neurol Scand. 2015;131:88-93.
12. American Association of Neuroscience Nurses. Care of the adult patient with a brain tumor. Chicago, IL: American Association of Neuroscience Nurses; 2014.
13. Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43:1678-1683.
14. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421-430.
15. Pardridge WM. Blood-brain barrier biology and methodology. J Neurovirol. 1999;5:556-569.
16. Temodar (temozolomide) product information. Whitehouse Station, NJ: Merck & Co; May 2015.
17. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
18. Van den bent MJ, Taphoorn MJ, Brandes AA, et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21:2525-2528.
19. Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11:6767-6771.
20. Liu R, Solheim K, Polley MY, et al. Quality of life in low-grade glioma patients receiving temozolomide. Neuro Oncol. 2009;11:59-68.
21. van den Bent MJ. Chemotherapy for low-grade glioma: when, for whom, which regimen? Curr Opin Neurol. 2015;28:633-638.
22. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30:3065-3070.
23. Rinne ML, Wen PY. Treating anaplastic oligodendrogliomas and WHO grade 2 gliomas: PCV or temozolomide? The case for temozolomide. Oncology (Williston Park). 2015;29:265,275.
24. Brandes AA, Nicolardi L, Tosoni A, et al. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol. 2006;8:253-260.
25. Lesser GJ. Chemotherapy of low-grade gliomas. Semin Radiat Oncol. 2001;11:138-144.
26. Hart MG, Garside R, Rogers G, et al. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013;(4):CD007415.
27. Chowdhary SA, Ryken T, Newton HB. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: a meta-analysis. J Neurooncol. 2015;122:367-382.
28. Gliadel (carmustine) product information. Atlanta, GA: Arbor Pharmaceuticals, LLC; November 2014.
29. Dixit S, Hingorani M, Achawal S, Scott I. The sequential use of carmustine wafers (Gliadel®) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg. 2011;25:459-469.
30. Brandes AA, Nicolardi L, Tosoni A, et al. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol. 2006;8:253-260.
31. Avastin (bevacizumab) prescribing information. South San Francisco, CA: Genentech, Inc; May 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






