US Pharm. 2010;35(4):HS-2-HS-8.
Many systemic diseases often have an ocular component that manifests secondarily. Patients with ocular manifestations may first present in the emergency department with relatively nonspecific symptoms such as visual disturbance or eye pain. Unfortunately, many ocular symptoms overlap in terms of the disease state they may be attributed to. In some cases, however, the information obtained from an ocular examination may aid in the differential diagnosis and appropriate management of the underlying disease. While there are a significant number of diseases known to present with ocular involvement, the aim of this article is to provide a general overview of the commonly encountered ocular manifestations of systemic disease in clinical practice and to review how they should be approached therapeutically.
Diabetic Retinopathy and Macular Edema
Diabetic retinopathy (DR), one of the leading causes of blindness worldwide among adults aged 20 to 74 years, is the most common microvascular complication of diabetes.1 It has been estimated that nearly all patients with type 1 diabetes and 60% of those with type 2 diabetes will develop some form of retinopathy within 20 years of disease onset regardless of their level of diabetic control.2 In patients with type 1 diabetes, retinopathy typically does not develop until 3 to 5 years after disease onset. In contrast, retinopathy may be the presenting manifestation of type 2 diabetes. Some studies estimate that 20% to 30% of patients with type 2 diabetes have evident retinopathy at the time of diagnosis.2
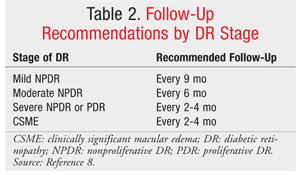
Retinopathy can precede nephropathy, making the early detection of ocular manifestations of diabetes essential. DR can be nonproliferative DR (NPDR) or proliferative (PDR), which is more advanced. DR is graded according to severity (mild, moderate, or severe). When observed during simple fundoscopic examination, mild NPDR is generally characterized by microaneurysms and small hemorrhages.1,3 In moderate-to-severe NPDR, increased vascular permeability, hard exudates, venous bleeding, and intraretinal microvascular anomalies are noted; these are caused by retinal ischemia. Macular edema is the principal mechanism of vision loss in DR, and it may be present in both NPDR and PDR (TABLE 1 and FIGURE 1). Macular edema is often treated with laser photocoagulation.4,5 (See FIGURE 1.)
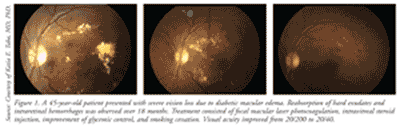
PDR is characterized by neovascularization (new vessel formation), which is a response to continued retinal ischemia.5 Neovascularization can cause vision loss due to vitreous hemorrhages and tractional retinal detachment, which can be treated by nonincisional surgery (laser photocoagulation) or incisional surgery (vitrectomy).4,5 There are two types of laser photocoagulation: focal and panretinal. Focal photocoagulation, which is used to treat macular edema, has been shown to reduce the risk of moderate vision loss by 50% to 70%.6 Panretinal photocoagulation, used to treat PDR, works by creating thousands of laser burns in areas of the retina away from the macula, causing abnormal vessels to shrink and thereby preventing them from hemorrhaging. This surgery can reduce the risk of moderate and severe vision loss by 50% in patients with PDR.6
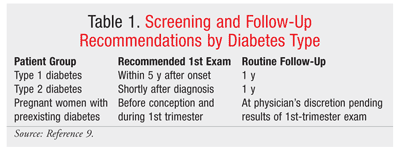
For patients already diagnosed with diabetes, follow-up with annual retinal screenings and prompt treatment are key components of patient care (see TABLES 1 and 2 for screening and follow-up recommendations).7,8 Adequate blood sugar control is paramount for preventing and controlling the progression of DR.
Several randomized, controlled clinical trials found that the combination of tight glycemic and blood-pressure control with laser photocoagulation therapy significantly prevents the progression of DR.7,9-11 The Diabetes Control and Complications Trial, which examined the effects of tight glycemic control on the incidence of DR, nephropathy, and neuropathy in type 1 diabetic patients, concluded that intensive glycemic control was associated with a 76% mean reduction in risk of retinopathy in patients with no retinopathy at baseline and with a 54% mean reduction in those with minimal-to-moderate NPDR at 36 months.10 The UK Prospective Diabetes Study (UKPDS) confirmed the protective effects of tight glycemic control in type 2 diabetic patients, finding a 25% reduction in microvascular complications in the intensive-therapy group.11 The UKPDS also found that every percentage-point decrease in glycosylated hemoglobin was associated with a 35% reduction in the risk of microvascular complications.11
Hypertension
Hypertension, which occurs in approximately 50 million people in the United States, remains a significant cause of morbidity and a leading cause of cardiovascular mortality.12,13 Hypertension affects the eye in a multitude of ways, including vascular injury and increased risk of embolic events (e.g., central retinal vein and branch retinal artery occlusion).
The ocular manifestations of hypertension are commonly classified as acute changes or chronic changes associated with long-term systemic hypertension.13 The perpetual increased pressure in the vasculature initiates a cascade of events resulting in a change in the physiology of the eye. This sequence of events, often collectively referred to as hypertensive retinopathy, is the most common manifestation.14 The differences in the composition of the vasculature and in anatomical location result in a different physiologic response to increased blood pressure. In the acute phase, hypertension predominantly affects the terminal arterioles; in chronic hypertension, the observed circulatory changes primarily involve the main retinal arterioles. Early manifestations include retinal hemorrhage, capillary obliteration, and macular edema attributable to hypertensive choroidopathy. Long-standing hypertension can result in the development of compensatory shunt vessels and arteriovenous nicking (impeded circulation in the retina).13
Hypertension also acts indirectly as a risk factor for other ocular diseases. These include retinal vein occlusion, retinal emboli, macroaneurysm, and optic neuropathy.
Hypertensive retinopathy is considered a reliable prognostic indicator of systemic disease. Perhaps the strongest correlation is between hypertensive retinopathy and risk of stroke. A study revealed that hypertensive retinopathy increased stroke risk two- to fourfold when patients with this condition were compared with patients who had no retinopathy.15 Studies also suggest that hypertensive retinopathy can be used to predict incident congestive heart failure, left ventricular hypertrophy, and renal impairment.13
The benefits of a fundoscopic examination are its ability to predict underlying systemic disease (typically cardiovascular in nature) and its ability to assess the need for initiating or modifying antihypertensive therapy. As with DR, disease-state control is of utmost importance in preventing and controlling progression of the ocular manifestations of hypertension. Blood pressure control, achieved through systemic pharmacotherapy and lifestyle modifications, is guided by the patient’s primary care provider.13
HIV/AIDS
Approximately 33 million people are infected with HIV worldwide.16 Opportunistic infections typically coincide with the patient’s CD4 count; thus, they often present when the CD4 count is ≤200 cells/mm3 or when the patient is unaware of HIV infection.16 Therefore, CD4 count remains the greatest predictor of ocular manifestations in HIV patients. It is not uncommon for an HIV patient to experience an ocular complication at some point in his or her lifetime. HIV infection places patients at risk for both infectious and noninfectious complications. The ocular manifestations of opportunistic infections are categorized based on anatomical origin (orbital/adnexal manifestations [external] versus anterior/posterior segment manifestations [internal]) or nonspecific systemic infection.
A myriad of infections are associated with the adnexa of the eye, including herpes zoster ophthalmicus (attributable to reactivation of varicella-zoster virus [VZV] infection in the first division of the fifth cranial nerve) and conjunctival microvascular disease, which affects up to 75% to 80% of patients.16 The most common manifestation is molluscum contagiosum, a viral infection of the skin. Lastly, up to 20% of patients who develop Kaposi’s sarcoma have ocular manifestations, usually with adnexal involvement.16
As many as 20% of patients may experience damage to the accessory and major lacrimal glands, resulting in keratoconjunctivitis sicca (dry eye).16 It should be noted that dry eye is a relatively nonspecific symptom and can have a number of causes, of which HIV is thought to be paramount. Anterior segment manifestations are often attributable to an infectious process, including infectious keratitis (bacterial, fungal, or protozoal in nature) and iridocyclitis (associated with cytomegalovirus [CMV] or VZV). Up to 50% of patients experience anterior uveitis, which may be caused by a number of different pathogens, including Treponema, VZV, and herpes simplex virus.16
Thirty percent to 50% of patients with decreased CD4 counts experience posterior segment manifestations, with the most common abnormalities being attributable to CMV and retinal microvasculopathy.16 CMV is the most common intraocular infection in AIDS patients. Ocular syphilis should be included in any differential diagnosis, as it mimics several diseases. Ocular syphilis in HIV-infected patients may present earlier and in an accelerated form.
Lastly, systemic infections such as cryptococcal meningitis or toxoplasmosis can have ophthalmic involvement. These types of infections, despite the eye not being the source of the infection, often result in nonspecific symptoms such as visual-field loss.17
Treatment is largely specific to the causative pathogen. Most manifestations of ocular disorders attributable to a viral infection respond to the appropriate antiviral (e.g., acyclovir, cidofovir, ganciclovir, valganciclovir).18 These patients are often managed by a physician specializing in infectious disease. Therapy is usually selected based on a global assessment of the patient that includes immune status and renal function. While a number of antivirals are available, use of these agents is not without systemic consequence, with the kidneys being most at risk for drug-induced harm. Herpesviruses usually are treated with acyclovir or the valine ester valacyclovir. Other agents, such as cidofovir, foscarnet, and ganciclovir, are typically reserved for cases of CMV retinitis or refractory herpes infections.18
Rheumatoid Arthritis
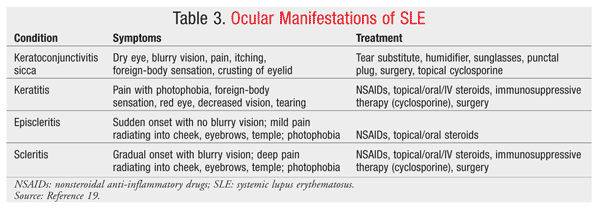
It is estimated that approximately 25% of patients with rheumatoid arthritis (RA) present with ocular manifestations.19 The most common external manifestation is keratoconjunctivitis sicca, which can be diagnosed using Schirmer’s test. The test evaluates the functionality of the lacrimal gland by measuring the amount of tear production through the placement of a Schirmer strip into the lower conjunctival cul-de-sac.
Episcleritis (superficial inflammation of the sclera) and scleritis (deeper inflammation of the sclera) are common ocular manifestations of RA, occurring in up to 25% and 10% of RA patients, respectively.19 Corneal disease is commonly associated with dry eye or with a form of anterior scleritis; it can include the presence of keratitis, sclerosing keratitis, and ulcerative keratitis. A combination of steroids, immunosuppressive therapy, and surgery often is necessary to prevent perforation of the cornea and permanent vision loss. Scleritis and episcleritis are distinguished based on anatomy and appearance. While the symptoms may be similar, pain is typically more severe in patients with scleritis. Application of phenylephrine 10% will help distinguish scleritis from episcleritis: Engorged vessels in episcleritis will blanch, while those in scleritis will not.19 In RA, episcleritis is more common than scleritis; however, scleritis tends to be a more destructive process, with necrotizing scleritis with inflammation being one presentation. It is important to differentiate scleritis from episcleritis, as studies have documented a higher mortality rate and wider spread of systemic disease in RA patients with scleritis.19
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a chronic, autoimmune, multisystem disease that affects the eyes in up to a third of patients. Ocular manifestations can be potentially sight-threatening and may be the presenting symptom of the disease. Ocular manifestations of SLE include external/anterior segment complications (usually associated with pain and redness) and posterior segment complications.
Common external eye complications in SLE include keratoconjunctivitis and discoid lupus erythematosus. Keratoconjunctivitis is the most common symptom associated with SLE, occurring in up to 30% of patients.19,20 Symptoms, clinical assessment, and treatment of dry eye are similar to those described in patients with RA (TABLE 3).19 Cyclosporine 0.05% is a commonly prescribed and effective treatment for dry eye syndrome. Eyelid disease presents with a discoid lupus-type rash over the eyelids that consists of raised, scaly lesions that respond well to systemic steroids and antimalarials such as hydroxychloroquine and chloroquine.
Posterior segment complications consist mainly of retinal disease. Retinopathy is present in 10% of patients with SLE, with signs correlating to the severity of systemic inflammation; it may indicate inadequate control of SLE.20 Some forms of retinopathy in SLE are similar to hypertensive retinopathy and DR, making management and monitoring difficult when these diseases occur simultaneously. Aggressive, severe retinopathy, in extreme cases, may lead to exudative retinal detachment, and treatment with systemic immunosuppression is necessary to control the disease.
Clinicians should be aware that agents used to treat SLE and RA may cause ophthalmic complications. The aminoquinolones chloroquine and hydroxychloroquine are antimalarials used to treatment SLE and RA. Chloroquine, and to a lesser extent hydroxychloroquine, can cause irreversible sight-threatening maculopathy at higher doses. Patients should undergo routine screening for retinal toxicity when receiving either of these drugs, as should patients who have been receiving therapy for more than 10 years or who have renal disease. Doses of chloroquine >3.5 mg/kg/day or doses of hydroxychloroquine >6.5 mg/kg/day significantly increase the risk of renal toxicity and should be monitored closely.21,22
REFERENCES
1. Abu El-Asrar AM, Al-Mezaine HS, Ola MS. Pathophysiology and management of diabetic retinopathy. Expert Rev Ophthalmol. 2009;4:627-647.
2. Dhanes T, Graham EM. Ocular disorders associated with systemic diseases. In: Riordan-Eva P, Whitcher JP, eds. Vaughan & Asbury’s General Ophthalmology. 17th ed. New York, NY: McGraw-Hill Medical; 2008: chapter 15.
3. Wipf JE, Paauw DS. Ophthalmologic emergencies in the patient with diabetes. Endocrinol Metab Clin North Am. 2000;29:813-829.
4. Mavrikakis E, Lam W-C, Khan BU. Macular edema, diabetic. http://emedicine.medscape.com/article/1224138-overview. Accessed January 27, 2010.
5. Gunderson CA, Karnath B. Retinal manifestations of diabetes mellitus and hypertension. Hosp Physician. 2003;39:15-18.
6. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
7. Fong DS, Aiello L, Gardner TW, et al. Diabetic retinopathy. Diabetes Care. 2003;26:S99-S102.
8. ONE Network. American Academy of Ophthalmology. Diabetic retinopathy PPP—September 2008. http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=d0c853d3-219f-487b-a524-326ab3cecd9a.
9. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
10. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
11. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet.
12. Dellacroce JT, Vitale A. Hypertension and the eye. Curr Opin Ophthalmol. 2008;19:493-498.
13. Oh KT, Hughes BM, Moinfar N. Hypertension. http://emedicine.medscape.com/article/1201779-overview. Accessed January 27, 2010.
14. Wong T, Mitchell P. The eye in hypertension. Lancet. 2007;369:425-435.
15. Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134-1140.
16. AIDS Epidemic Update December 2009. Geneva, Switzerland: UNAIDS/WHO; 2009. http://data.unaids.org/pub/Report/2009/2009_epidemic_update_en.pdf. Accessed February 20, 2010.
17. Moraes HV. Ocular manifestations of HIV/AIDS. Curr Opin Ophthalmol. 2002;13:397-403.
18. Copeland R, Phillpotts BA. Ocular manifestations of HIV. http://emedicine.medscape.com/article/1216172-overview. Accessed January 27, 2010.
19. Patel SJ, Lundy DC. Ocular manifestations of autoimmune diseases. Am Fam Physician.
20. Sivaraj RR, Durrani OM, Denniston AK, et al. Ocular manifestations of systemic lupus erythematosus. Rheumatology. 2007;46:1757-1762.
21. Chloroquine hydrochloride and chloroquine phosphate. In: McEvoy GK, Miller J, Litvak K, eds. AHFS Drug Information 2004. Bethesda, MD: American Society of Health System Pharmacists; 2004:822-827.
22. Hydroxychloroquine sulfate. In: McEvoy GK, Miller J, Litvak K, eds. AHFS Drug Information 2004.
Bethesda, MD: American Society of Health System Pharmacists; 2004:827-828.
To comment on this article, contact rdavidson@uspharmacist.com.






