US Pharm. 2015;40(6):HS19-HS24.
ABSTRACT: Systemic administration of drugs can affect all structures and functions of the eye. These agents can cause several common and distinct adverse ophthalmic reactions. Ocular adverse effects may be related to the pharmacodynamic or pharmacokinetic action of a drug, possibly even serving as a marker of toxicity. Both temporary visual disturbances and permanent vision loss are possible. Recommendations for monitoring and treatment of ophthalmic toxicities can be found in clinical practice guidelines and prescribing information. The pharmacist can have a role in the management and reporting of these adverse events.
The eye, responsible for vision, is made up of several key parts including the cornea, lens, retina, iris, pupil, and optic nerve. The cornea, which covers the front of the eye, is the first place light enters the eye. The iris—the pigmented part of the eye that determines individual eye color—dilates and contracts the pupil, regulating the amount of light that enters the eye. The lens focuses the light onto the retina. Rods and cones are cells in the retina that transmit light via electrical signaling to the brain, by way of the optic nerve, for processing. Cones are responsible for color vision and sight in bright light, while rods are helpful for sight in low light.1,2
The eye is coated in aqueous humor to maintain intraocular pressure and serve as protection. Aqueous humor drains through Schlemm’s canal. If there is a disturbance in the drainage of this fluid, glaucoma can result. Drugs that modify the production or drainage of aqueous humor can potentiate or mitigate glaucoma.1,2
Contraction of the pupil leads to miosis, or a reduction in pupil size. Miosis typically occurs in situations of bright light exposure and is controlled by the parasympathetic nervous system. Mydriasis is an expansion or dilation of the pupil and is controlled by the sympathetic nervous system. Mydriasis occurs in the dark to help increase sight. Anticholinergic activity leads to mydriasis, while cholinergic activity leads to miosis.1,2
Systemic Drug Therapy
Drugs can affect mydriasis and miosis. Atropine is commonly given at ophthalmologist visits to induce mydriasis and cycloplegia. Atropine is extracted from the deadly nightshade plant, Atropa belladonna, and was used historically to induce mydriasis for beauty. Other agents used to induce mydriasis include phenylephrine and cyclopentolate. Use of cholinergic agents such as pilocarpine would cause miosis.1,2
Systemic administration of drugs can affect all structures and functions of the eye. The pharmacologic or pharmacokinetic properties of a drug can cause expected ocular adverse effects, possibly even serving as a marker of toxicity. Both permanent vision loss and temporary visual disturbances are possible. Pharmacists should be aware of common and severe adverse events and the reporting mechanisms. Recommendations for monitoring and management of ocular effects vary in the literature. A number of organizations have published clinical practice guidelines on the monitoring parameters for some of the agents, including the American Optometrist Association (AOA).3 Prescribing information for individual agents should also be consulted for monitoring recommendations. This article will focus on a group of medications that can cause several common and distinct adverse ophthalmic reactions, although it is not a complete compilation of all such agents (TABLE 1).
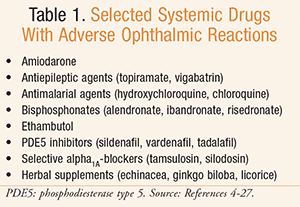
Amiodarone
Used for the treatment of various arrhythmias, amiodarone is designated a class III anti-arrhythmic, but exhibits effects of all four classes. Amiodarone has been associated with a range of ocular toxicities, the most common being corneal deposits and colored halos around lights.4 Although rare, optic neuropathy has also been seen with amiodarone therapy. Amiodarone is generally used for long-term therapy for various cardiac arrhythmias. Typical dosing is 200 to 400 mg daily. It is lipid soluble, allowing it to reach many tissues in the body, and has a long half-life of approximately 40 to 55 days.5
Corneal deposits or keratopathy with amiodarone therapy is both time- and dose-dependent and is seen in 69% to 100% of patients.6 Amiodarone keratopathy occurs in three stages after at least 1 month of treatment.4 The first stage is described as the appearance of a horizontal line in the cornea in patients on dosages of 200 to 400 mg. Higher daily doses can result in stage 2 and 3 effects, which are characterized as a cat-whisker pattern and a whorl-like (verticillata) pattern in the cornea, respectively.7 Formation of deposits seems to be a result of complexes formed with the drug and phospholipids that cannot be metabolized. The deposits usually occur bilaterally and normally do not result in visual impairment; however, the colored halos appearing around lights may be linked to these deposits. Following amiodarone discontinuation, it can take from 3 to 20 months for the deposits to completely resolve.4
Optic neuropathy occurs less frequently (<2%) with amiodarone administration, but can be severe, leading to vision loss.6 Amiodarone-associated optic neuropathy has a gradual onset with slow progression that can ultimately result in bilateral vision loss and disc swelling. The exact mechanism is unknown, but may be related to accumulation in optic nerve axons. If optic neuropathy is suspected, discontinuation of amiodarone should be considered, if possible, as visual loss can be permanent.6
Cases of eyelid irritation, eyelid cysts, and dry eyes have also been seen with amiodarone therapy.4 Due to the high frequency of ocular toxicity with amiodarone, it is recommended that patients receive a baseline ophthalmic examination prior to initiation of therapy, with repeat examinations every 6 months in the first year and every 12 months thereafter.3,6
Antiepileptic Agents
Topiramate can be used as adjunctive or monotherapy for the treatment of seizures. It can also be used for migraine prophylaxis. Topiramate has been reported to cause a plethora of ocular adverse effects including acute angle-closure glaucoma with elevations in intraocular pressure. Acute angle-closure glaucoma is likely due to swelling of the ciliary body.7,8
Acute angle-closure glaucoma is most likely to occur within the first 2 weeks of drug initiation or dose titration. Generally, acute angle-closure glaucoma presents with a specific onset of bilateral blurry vision.8,9 If patients receive topiramate for migraine prophylaxis, they should be cognizant that this sign of toxicity may overlap with signs and symptoms of a migraine. Pharmacists, especially at the time of dispensing of a new prescription, can warn patients to report any change in vision, particularly during the first few weeks of therapy. If visual changes are noticed, topiramate should be discontinued to avoid the risk of permanent visual loss, and the patient should be referred to an ophthalmologist.8,9 Topical application of mydriasis-inducing agents and beta blockers may be useful to lower intraocular pressure by retracting the ciliary processes.9
Vigabatrin can be used for the treatment of refractory seizures in patients who have not responded to other antiepileptic drug therapy. A black box warning exists due to the risk of progressive and permanent bilateral concentric visual-field constriction and potential reduction of visual acuity.10 Approximately 30% to 40% of patients have reported visual loss associated with vigabatrin therapy. Risk for vision loss is related to duration (longer than 6 months) and dosage; however, no specific time or dose of vigabatrin has been found to be without risk.11
SHARE, a Risk Evaluation and Mitigation Strategy (REMS) program, exists for the use of vigabatrin because of the risk of ophthalmic toxicity; it ensures that providers are certified prior to use of vigabatrin in patients and understand the monitoring required. If the decision is made to initiate vigabatrin, patients must have ophthalmic examinations at baseline and at least every 3 months thereafter.11
If patients exhibit signs of ophthalmic toxicity, consideration should be given to discontinuing vigabatrin therapy. Because of the high risk of ocular adverse effects, vigabatrin should also be discontinued if patients being treated for refractory seizures do not exhibit clinical benefit within 3 months of treatment initiation. Visual examinations should continue for at least 3 to 6 months following discontinuation of therapy.10
Antimalarial Agents
The antimalarial agents hydroxychloroquine and the less common chloroquine possess anti-inflammatory, antithrombotic, and immunomodulatory effects. Hydroxy-chloroquine is used in the treatment of various conditions including malaria and, more commonly in the United States, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Typical dosages of hydroxychloroquine are 200 to 400 mg daily for SLE and RA, with patients receiving these agents indefinitely.12
Both chloroquine and hydroxychloroquine carry a risk for ophthalmic toxicity. The mechanism of toxicity is not well understood. The drugs affect the metabolism of retinal cells and bind to melanin in the retinal pigment epithelium; however, it is unclear if this leads to the ophthalmic toxicity.13 In early stages of toxicity, visual acuity may be unchanged with some minor reading difficulties. As exposure to the drug continues, a loss of visual acuity and vision loss may occur. Toxicity from chloroquine and hydroxychloroquine is characterized by bilateral bull’s-eye maculopathy seen on ophthalmic examination.13
Several risk factors have been identified to place patients at a higher risk for hydroxychloroquine retinopathy. As treatment duration approaches 5 to 7 years, the risk for retinopathy approaches 1%.13 Cumulative doses of 1,000 g or daily doses above 400 mg per day also lead to a greater risk for toxicity. Since the kidney and liver are responsible for metabolism and excretion, patients with underlying renal or hepatic disease are at higher risk for toxicities from increased drug levels. Elderly patients and those with preexisting retinal and macular disease are believed to be at higher risk since they may be more prone to developing ophthalmic complications.13
Currently, there is no treatment for hydroxychloroquine-induced retinopathy. Discontinuation can help stop progression, but damage may not be reversible. Screening and risk evaluation are the key to limiting ocular toxicity. Prior to starting hydroxychloroquine, patients should undergo a baseline ophthalmic examination. The time frame for repeat examinations varies, with the prescribing information indicating 3-month monitoring, The AOA recommends 6-month monitoring, and other sources recommend annual examinations after 5 years of treatment.3,12,13 The use of an objective examination as well as the visual-field test and ophthalmic examination are recommended.12,13 AOA-specific recommendations for screening can be found in the clinical guidelines.3
Bisphosphonates
Bisphosphonates (e.g., alendronate, ibandronate, and risedronate) are used for osteoporosis management in men and postmenopausal women, as well as in other bone disorders such as Paget disease. Bisphosphonates exhibit their effect by mimicking an endogenous bone resorption inhibitor.14
Reported cases of ocular adverse events are rare and were not seen in the large clinical trials that led to each agent’s approval.15 Inflammatory eye reactions (IERs) following bisphosphonate use have been reported in case reports and case series.15 Inflammatory reactions include conjunctivitis, uveitis, scleritis, episcleritis, and keratitis. Although the exact mechanism of toxicity is unknown, it may be a result of bisphosphonate secretion into tears. This secretion leads to irritation of mucous membranes and cytokine release.15
Treatment of bisphosphonate IER varies depending on the type and extent of inflammation. If inflammation is associated with vision loss or pain, the patient should be referred to an ophthalmologist.16 Most cases of conjunctivitis resolve without treatment. In rare cases, an ophthalmologically administered nonsteroidal anti-inflammatory drug (NSAID) may be needed to control inflammation. Uveitis usually requires ocular or systemic medication for treatment and may require discontinuation of the bisphosphonate. In cases of episcleritis, ocular medication may be needed but the bisphosphonate can be continued. If scleritis occurs, bisphosphonate discontinuation is required for symptom resolution.16
Ethambutol
Used in the treatment of tuberculosis, ethambutol works by inhibiting synthesis of metabolites and impairing cell metabolism in susceptible mycobacteria. It is often used as part of a four-drug combination with isoniazid, rifampin, and pyrazinamide. Dosing of ethambutol varies depending on the regimen and ranges from 15 to 25 mg/kg for daily dosing strategies to 50 mg/kg twice weekly. Ethambutol is typically used for 8 weeks in the treatment of tuberculosis.17 Several ocular adverse events are associated with the use of ethambutol, the most severe being optic neuritis.7 Other ocular events reported include color vision changes and visual-field defects.
The exact mechanism for ethambutol-induced optic neuropathy is unknown. A metabolite of ethambutol chelates copper and decreases the amount available, therefore decreasing the axonal transport around the optic nerve, potentially contributing to the neuropathy.7
Ocular toxicity is dosage-related, with patients receiving higher doses at increased risk. Optic neuropathy can be seen in up to 50% of patients receiving doses of 60 to 100 mg/kg/day.7 The use of typical dosing decreases the incidence to 5% to 6% with doses of 25 mg/kg/day and 1% with doses 15 mg/kg/day. Patients who receive ethambutol for a longer period of time are also at higher risk. Visual problems generally appear after 4 to 12 months of treatment, much after the normal duration of use for most patients.18
Ethambutol is cleared renally, by both glomerular filtration and tubular secretion; thus, renal impairment can lead to increased drug levels and the development of toxicity. There has also been a correlation between increasing age and development of toxicity, which could be due to decreased clearance with age-related renal function declines.19
Baseline visual acuity measurements and color discrimination testing are recommended for all patients. Monthly ophthalmic exams may be indicated for patients who receive dosages above 15 mg/kg/day or patients who will receive ethambutol for over 2 months.3,17 Patients with additional risk factors for ophthalmic problems, such as renal insufficiency, should be examined monthly. Patients should be aware of the risk for ophthalmic toxicity when ethambutol is initiated and be advised to report any visual disturbances to their physician immediately. Ethambutol should be discontinued if any visual symptoms occur.3,17
Phosphodiesterase Inhibitors
The phosphodiesterase type 5 (PDE5) inhibitors sildenafil, vardenafil, and tadalafil are effective for the treatment of erectile dysfunction through inhibition of cyclic guanosine monophosphate.20 Daily use of tadalafil also has an indication for benign prostatic hyperplasia (BPH). PDE5 inhibitors have been associated with several ocular adverse events, most commonly changes in color perception and blurred vision.7
Ocular events are related to inhibition of both PDE5, in the retina, and PDE6, located in photoreceptors of the rods and cones.21,22 Sildenafil can lead to 10% inhibition of PDE6, and all drugs in this class have similar ocular effects.20 Some reported events have been classified as “certain” dose-dependent effects, including a change in color perception (usually blue or green), blurred vision, changes in light perception, photophobia, and eye pain. The onset of ocular events normally starts within 15 to 30 minutes and peaks 60 minutes after administration.7 Based on current evidence, these events have not led to permanent changes in the perception of color.21,22
Cases of anterior ischemic optic neuropathy have also been reported following the use of PDE5 inhibitors; however, there are no data to confirm a certain relationship, so it is listed as a “possible” effect.7,20 Patients who previously experienced nonarteritic anterior ischemic optic neuropathy should not take PDE5 inhibitors, as they may be at higher risk to develop it again.
Based on available data from postmarketing surveillance, ocular adverse events from this class are generally benign and transient.7
Tamsulosin and Selective Alpha1A-Blockers
Tamsulosin is a selective alpha1A antagonist commonly used for the treatment of BPH. Tamsulosin and other selective alpha1A antagonists, such as silodosin, have been associated with intraoperative floppy iris syndrome (IFIS).23 IFIS, to a lesser extent, has also been associated with nonselective alpha antagonists.24 Dilation of the pupil is necessary to reduce the risk of complications during cataract surgery and is achieved with the use of mydriatic agents. Blockade of alpha1A receptors causes a relaxation of the iris dilator, rendering the agents used during the procedure ineffective.23,25 Complications that may result include iris prolapse, pupillary miosis, iris trauma, iris aspiration, and vitreous loss.
IFIS is seen mainly during cataract surgery and to a lesser extent in glaucoma surgery.23 In 2010, there were over 24 million cataract surgeries, with the number expected to grow to almost 39 million by the year 2030.26 Although alpha-blockers are used primarily in men for BPH, women can still be at risk for developing IFIS, as these agents can be used for the treatment of other bladder problems and hypertension.25
It is important to know if alpha-blockers are used prior to cataract surgery, since discontinuation has not been effective in preventing IFIS and is not a recommended strategy.25 Use of atropine drops or epinephrine dilution can be considered as prophylaxis prior to surgery. Application of atropine 1% drops has shown success in providing mydriasis, but patients often require another intervention. Intracameral administration of diluted epinephrine during the procedure is the recommended prophylactic strategy.25 Several non-pharmacologic interventions may be used during the procedure to reduce the risk of IFIS-including triplanar corneal incisions, trypan blue, less aggressive irrigation and aspiration, iris retractors, and refractor rings.25
Herbal Supplements
Ocular adverse events have also been reported with herbal supplements. These events have been collected retrospectively based on observational cases reported to various agencies.6,27
Niacin, commonly used for its cholesterol-lowering effects, is the herbal with the most reported ocular events. Cystoid macular edema has been reported with daily doses as low as 1.5 g. The edema typically resolves within 2 weeks of discontinuation. Cases of blurred vision have also been seen following the use of niacin.6,27
Chamomile, used topically around the eye, can cause severe conjunctivitis and angioedema likely due to sensitivity to allergens found in chamomile. Echinacea, used orally for immune-boosting effects, can cause eye irritation and conjunctivitis. Symptom resolution occurs within a day after stopping use. Ginkgo biloba, used orally for memory, has resulted in hyphema and retinal hemorrhages, likely linked to the inhibition of platelet aggregation. Because of this bleeding risk, caution should be used in patients on concomitant anticoagulants or antiplatelet agents. Licorice, used orally for anti-inflammatory and antiplatelet effects, has been associated with cases of transient visual loss following consumption of large quantities. The symptoms reported were similar to those of an ocular migraine.6,27
Conclusion
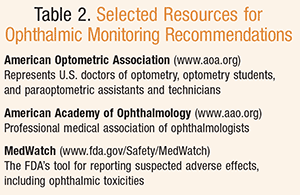
Ocular adverse effects can occur with systemically administered agents. These events can be related to the pharmacodynamic or pharmacokinetic action of the drug, or could be unrelated to either. Recommendations for monitoring can be found in various organizations’ clinical practice guidelines, including the American Academy of Ophthalmology and the AOA (TABLE 2), as well as in prescribing information for individual medications. Ophthalmic adverse drug reactions should be reported to the FDA through the FDA Adverse Event Reporting System (FAERS) and MedWatch, which are accessible to healthcare practitioners and the public.28
Pharmacists should be aware of systemic agents that can cause ophthalmic toxicity. They are positioned to discuss the potential for ophthalmic toxicity with systemically administered agents and to stress the importance of maintaining frequent monitoring and follow-up for adverse visual effects.
REFERENCES
1. Horton JC. Disorders of the eye. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.2. Henderer JD, Rapuano CJ. Chapter 64. Ocular pharmacology. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011.
3. Gurwood AS. Optometric clinical practice recommendations for monitoring ocular toxicity of selected medications. American Optometric Association. www.aoa.org/Documents/optometrists/QI/optometric-clinical-practice-recommendations-for-monitoring-ocular-toxicity-of-selected-medications.pdf. Accessed May 13, 2015.
4. Mantyjarvi M, Tuppurainen K, Ikaheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998;42(4):360-366.
5. Cordarone (amiodarone) package insert. Philadelphia, PA: Pfizer Inc; March 2015.
6. Santaella R, Fraunfelder FW. Ocular adverse effects associated with systemic medications: recognition and management. Drugs. 2007;67(1): 75-93.
7. Kaplan L, Cappaert WE. Amiodarone keratopathy. Correlation to dosage and duration. Arch Ophthalmol. 1982;100(4):601-602.
8. Abtahi M, Abtahi S, Fazel F, et al. Topiramate and the vision: a systematic review. Clin Ophthalmol. 2012;6:117-131.
9. Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology. 2004;111(1): 109-111.
10. Sabril (vigabatrin) package insert. Deerfield, IL: Lundbeck; October 2013.
11. Kraus GL. Evaluating risks for vigabatrin treatment. Epilepsy Curr. 2009;9(5):125-129.
12. Hydroxychloroquine package insert. New York, NY: Kanetta Pharmacal; April 2002.
13. Marmor M, Kellner U, Lyons JS, et al; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415-422.
14. Fosamax (alendronate) prescribing information. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
15. Clark EM, Durup D. Inflammatory eye reactions with bisphosphonates and other osteoporosis medications: what are the risks? Ther Adv Musculoskelet Dis. 2015;7(1):11-16.
16. Fraunfelder FW, Fraunfelder FT. Adverse ocular drug reactions recently identified by the National Registry of Drug-Induced Ocular Side Effects. Ophthalmology. 2004;111(7):1275-1279.
17. American Thoracic Society, CDC, Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1-77.
18. Grzybowski A, Zulsdorff M, Wilhelm H, Tonagel F. Toxic optic neuropathies: an updated review. Acta Ophthalmologica. 2014 Aug 27:1-9 [Epub ahead of print].
19. Talbert Estlin KA, Sadun AA. Risk factors for ethambutol optic toxicity. Int Ophthalmol. 2010;30(1):63-72.
20. Fraunfelder FW. Visual side effects associated with erectile dysfunction agents. Am J Ophthalmol. 2005;140(4):723-724.
21. Marmor MF, Kessler R. Sildenafil (Viagra) and ophthalmology. Surv Ophthalmol. 1999;44(2):153-162.
22. Kerr NM, Danesh-Meyer HV. Phosphodiesterase inhibitors and the eye. Clin Experimental Ophthalmol. 2009;37(5):514-523.
23. Friedman AH. Tamsulosin and the intraoperative floppy iris syndrome. JAMA. 2009;301(19):2044-2045.
24. Issa SA, Hadid OH, Baylis O, et al. Alpha antagonists and intra-operative floppy iris syndrome: a spectrum. Clin Ophthalmol. 2008;2(4): 735-741.
25. Janjua S, Cremers S. Managing intraoperative floppy iris syndrome. Eyenet. May 2009. http://development.aao.org/publications/eyenet/200905/pearls.cfm. Accessed February 17, 2015.
26. National Eye Institute. Facts about cataracts. www.nei.nih.gov/eyedata/cataract#3b. Accessed February 17, 2015.
27. Fraunfelder FW. Ocular side effects from herbal medicines and nutritional supplements. Am J Ophthalmol. 2004;138(4):639-647.
28. FDA Adverse Event Reporting System (FAERS). www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/. Accessed February 20, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






