Approximately 70% of children feel fear, stress, or anxiety prior to a needlestick procedure or venipuncture.1 Topical anesthetics are safe and effective for reducing the physical and emotional distress children may experience during these procedures, and they have been utilized for children in the emergency-department (ED) setting since the 1980s.2 Although types of topical anesthetics have not changed considerably over the last few decades, several advances have been made in the technologies available for drug delivery.
Topical anesthetics reduce pain by inhibiting the transduction and transmission of nerve impulses.3 This change occurs secondary to an alteration in transmission through voltage-sensitive sodium channels, resulting in a rise in the action-potential threshold. Traditional agents utilized as topical anesthetics for pediatric needlestick procedures include eutectic mixture of local anesthetics (EMLA), various lidocaine formulations, and vapocoolants. Newer agents and novel drug-delivery systems include lidocaine/tetracaine heating patches and pressurized lidocaine delivery systems (J-Tip, Zingo). This article will review the pharmacologic formulations available for use during venipuncture, needlestick procedures, and venous cannulation; it also will highlight their efficacy and safety when used in the pediatric population.
Eutectic Mixture of Local Anesthetics
EMLA contains lidocaine 2.5% and prilocaine 2.5% as an oil-in-water emulsion.4 By definition, a eutectic mixture has a melting point that is lower than either constituent alone; therefore, this mixture of lidocaine and prilocaine in a 1:1 ratio is present in liquid phase. EMLA is available commercially as cream, gel, and anesthetic disc. The cream and the disc are approved for topical use in children undergoing minor (e.g., venipuncture) and major (e.g., skin-graft harvesting) dermal procedures. The cream can be used in either setting, whereas the disc should be used only during minor procedures because of its inability to cover more than a limited surface area (up to 40 cm2).
A concern with EMLA is the risk of systemic absorption; however, absorption is minimal and should not produce toxic plasma levels with routine dosing, even when applied to open wounds.4 When applied to the skin, an anesthetic response occurs within 45 to 60 minutes and lasts for one to two hours after removal. Additionally, the site of application can affect the duration of analgesia; areas with increased vasculature can increase drug clearance.3 Dosing for EMLA is age- and weight-based, and the manufacturer recommends the following: age 0-3 months or <5 kg should receive 1 g over 10 cm2 skin for up to 1 hour; age 3-12 months and >5 kg should receive 2 g over 20 cm2 skin for up to 4 hours; age 1-6 years and >10 kg should receive 10 g over 100 cm2 skin for up to 4 hours; and age 7-12 years and >20 kg should receive 20 g over 200 cm2 skin for up 4 hours.4 For maximum skin absorption, EMLA should be applied beneath occlusive dressings such as Tegaderm.5 Because of the risk of methemoglobinemia, EMLA use should be avoided in neonates with a gestational age of less than 37 weeks and in infants under the age of 1 year who are receiving concomitant therapy with agents such as phenytoin or acetaminophen.4
EMLA has demonstrated efficacy in reducing pain in infants and children who have undergone procedures such as venipuncture. In a trial of chronically ill infants and toddlers, mean visual analog pain scale scores were significantly lower with the use of EMLA cream versus placebo (P =.01).6 When serum methemoglobin was monitored, levels were similar in both groups and did not increase beyond normal range. Another trial evaluating pain response and ease of venipuncture found that the 20 children who received EMLA had lower pain scores (P ≤.01) and significant improvements in ease of venipuncture (P ≤.05).7
Topical Liposomal Lidocaine Formulations
LMX-4 is an OTC topical liposomal formulation of 4% lidocaine that was previously known as ELA-Max.3 It is rapidly absorbed through the skin and within 20 to 30 minutes produces an anesthetic effect that is sustained for up to 1 hour after application. LMX-4 is FDA-approved for the temporary relief of pain and pruritus associated with minor burns, cuts, and insect bites. The area of LMX-4 application should be no larger than 100 cm2 in children weighing ≤10 kg or between 10 kg and 20 kg for each use, because the amount of absorption depends upon the duration and surface area of the application.
When utilized in a clinical setting, LMX-4 was as effective as EMLA for alleviating pain from venipuncture and for reducing distress scores on an observer-rated behavioral scale.8 In children undergoing peripheral IV catheter insertion, LMX-4 was similar to buffered lidocaine for reducing pain and anxiety as rated by the patient, a parent, and a blinded observer.9 Furthermore, LMX-4 produced anesthesia within 30 minutes without the need for occlusive dressing, compared with 60 minutes for EMLA and its dressing.9,10 Unlike EMLA, LMX-4 has not been found to induce methemoglobinemia and produces only minor local effects, such as blanching and erythema. In summary, LMX-4 has a faster onset of anesthesia and a more favorable adverse-effect profile than EMLA, and needs no prescription.
Vapocoolants
Vapocoolants have long been used to control minor pain associated with topical procedures. Ethyl chloride (Gebauer's Ethyl Chloride) is indicated for pain control of injections and minor topical procedures and for temporary relief of minor sports injuries.11 Studies of ethyl chloride as a topical anesthetic have been small, however, and data on efficacy are variable.12,13 Overall, its use has decreased given the wide variety of products now available, and some children may not like its cooling effect. For delivery of preinjection anesthesia, Gebauer's Ethyl Chloride should be sprayed continuously for four to 10 seconds, approximately three to seven inches from the skin.11 Needle insertion should occur immediately after application of the product.
Heat-Enhanced Drug-Delivery Systems
With their ease of application, patches are another method of topical anesthesia that is favored for use in children. One novel product consists of a eutectic mixture of lidocaine 70 mg and tetracaine 70 mg (Synera). This patch is a disposable, oxygen-activated system that uses heat to enhance drug absorption through the stratum corneum.14 It was originally marketed without the heating element under the trade name S-Caine Patch. The Synera patch is specifically indicated for use on intact skin for superficial venous access or minor dermatologic procedures in patients aged 3 years and older.14 Application is recommended for 20 to 30 minutes prior to venous cannulation. To avoid systemic toxicity, lidocaine/tetracaine patches should not be applied to broken or inflamed skin or to mucous membranes. Multiple patches should not be used simultaneously or in immediate succession. Synera is contraindicated in patients with known hypersensitivity to para-aminobenzoic acid. Studies have consistently produced favorable results, demonstrating that the lidocaine/tetracaine warming patch significantly reduced pain in children during IV cannulation versus placebo, with no effect on the number of successful cannulation attempts.15,16
Needle-Free Pressurized Delivery Systems
Two single-use, needle-free anesthesia delivery systems have been introduced that allow for the rapid delivery of lidocaine hydrochloride prior to peripheral venous access procedures. The J-Tip device, introduced in 2001, uses compressed carbon dioxide for drug delivery into the subcutaneous space. It was initially designed for the needle-free delivery of insulin in patients with diabetes.17 Currently, J-Tip is a delivery system only and must be filled by the user with the desired drug product (typically 1% or 2% buffered lidocaine powder). Introduced in 2007, Zingo is a prefilled syringe of sterile lidocaine powder 0.5 mg that uses pressurized helium to enable the drug to penetrate the epidermis.18
J-Tip and EMLA were compared in a head-to-head trial examining their efficacy in reducing pain during IV cannulation and placement.19 Patients aged 7 to 19 years were randomized to receive 1% buffered lidocaine 0.25 mg via J-Tip (n = 57) or EMLA 2.5 g (n = 59) prior to cannulation. Pain was rated via visual analog scale scores (1-10) at the time of venous cannulation. Median pain score was 0 in the J-Tip group and 3 in the EMLA group (P =.0001). Although 40% of patients in the EMLA group had product-application times of less than the 60-minute recommendation, a subgroup analysis of those with proper EMLA administration further supported the superiority of J-Tip.
One phase III study examined venipuncture and venous cannulation pain after the use of Zingo in approximately 600 children aged 3 to 18 years.20 This randomized, double-blind trial compared the delivery of lidocaine powder 0.5 mg via Zingo versus a sham placebo. Patients randomized to Zingo had significantly improved pain control compared with the placebo group. Twelve patients in the Zingo group experienced mild treatment-related adverse effects, including contusion and erythema.
The Role of the Pharmacist
Pharmacists can contribute significantly to several areas surrounding the use of topical anesthesia. First, the pharmacist can make sure that topical anesthesia is used when necessary. Some practitioners suggest that pain control should always be used when venipuncture or venous access is performed in children and that failure to offer topical pain control can lead to bad memories, anxiety, and needle phobia.21 A study of more than 1,200 subjects in a pediatric ED found that very few patients undergoing venipuncture received any pain management during or after the procedure, however.22 Second, the pharmacist can see to it that topical anesthetics and drug-delivery products are used at an appropriate dose and duration to ensure maximal efficacy while avoiding systemic absorption. The FDA recently issued a public-health advisory to reiterate the importance of using these agents sparingly and at the lowest dose possible.23 Finally, the pharmacist should be involved in patient drug counseling and monitoring for adverse effects, including signs of systemic toxicity (neurotoxicity, cardiotoxicity, and methemoglobinemia).24
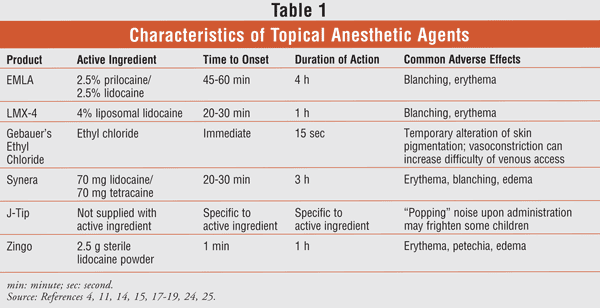
Conclusion
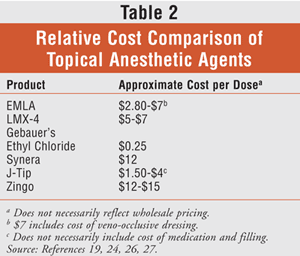
There are many options for the application and delivery of topical anesthesia in children, and most of them are safe and effective when used appropriately (TABLE 1). Choice of product may be narrowed down to patient and provider preference, ease of administration, or cost (TABLE 2). While the product used during the delivery of topical anesthesia prior to peripheral venous access procedures varies by institution, care should always be taken to ensure appropriate pain control in pediatric patients.
REFERENCES
1. American Pain Foundation. Pain facts: overview of American pain surveys.
www.painfoundation.org/page.
2. Pryor GJ, Kilpatrick WR, Opp DR. Local anesthesia in minor lacerations: topical TAC vs lidocaine infiltration. Ann Emerg Med. 1980;9:568-571.
3. Tadicherla S, Berman B. Percutaneous dermal drug delivery for local pain control. Ther Clin Risk Manag. 2006;2:99-113.
4. EMLA (eutectic mixture of lidocaine and prilocaine) package insert. Wilmington, DE: AstraZeneca; 2005.
5. Lycka BA. Medical indications for using a topical anesthetic. Perspect Pain Manage. 1991;1:9-12.
6. Robieux I, Eliopoulos C, Hwang P, et al. Pain perception and effectiveness of the eutectic mixture of local anesthetics in children undergoing venipuncture. Pediatr Res. 1992;32:520-523.
7. Cooper CM, Gerrish SP, Hardwick M, Kay R. EMLA cream reduces the pain of venepuncture in children. Eur J Anaesthesiol. 1987;4:441-448.
8. Eichenfield LF, Funk A, Fallon-Friedlander S, Cunningham BB. A clinical study to evaluate the efficacy of ELA-Max (4% liposomal lidocaine) as compared with eutectic mixture of local anesthetics cream for pain reduction of venipuncture in children. Pediatrics. 2002;109:1093-1099.
9. Luhmann J, Hurt S, Shootman M, Kennedy R. A comparison of buffered lidocaine versus ELA-Max before peripheral intravenous catheter insertions in children. Pediatrics. 2004;113:217-220.
10. Kleiber C, Sorenson M, Whiteside K, et al. Topical anesthetics for intravenous insertion in children: a randomized equivalency study. Pediatrics. 2002;110:758-761.
11. Gebauer's Ethyl Chloride (ethyl chloride) product sheet. Cleveland, OH: Gebauer Company; 2005.
12. Soueid A, Richard B. Ethyl chloride as a cryoanalgesic in pediatrics for venipuncture. Pediatr Emerg Care. 2007;23:380-383.
13. Costello M, Ramundo M, Christopher NC, Powell KR. Ethyl vinyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clin Pediatr (Phila). 2006;45:628-632.
14. Synera (lidocaine 70 mg/tetracaine 70 mg) package insert. Chadds Ford, PA: Endo Pharmaceuticals; 2006.
15. Sethna NF, Verghese ST, Hannallah RS, et al. A randomized controlled trial to evaluate S-Caine patch for reducing pain associated with vascular access in children. Anesthesiology. 2005;102:403-408.
16. Singer AJ, Taira BR, Chisena EN, et al. Warm lidocaine/tetracaine patch versus placebo before pediatric intravenous cannulation: a randomized controlled trial. Ann Emerg Med. 2008;52:41-47.
17. J-Tip Needle Free Injection System product information. Irvine, CA: National Medical Products, Inc; 2001.
18. Zingo (lidocaine) package insert. South San Francisco, CA: Anesiva, Inc; 2007.
19. Jimenez N, Bradford H, Seidel KD, et al. A comparison of a needle-free injection system for local anesthesia versus EMLA for intravenous catheter insertion in the pediatric patient. Anesth Analg.
20. Zempsky WT, Bean-Lijewski J, Kauffman RE, et al. Needle-free powder lidocaine delivery system provides rapid effective analgesia for venipuncture or cannulation pain in children: randomized, double-blind comparison of venipuncture and venous cannulation pain after fast-onset needle-free powder lidocaine or placebo treatment trial. Pediatrics. 2008;121:978-987.
21. Kennedy RM, Luhmann J, Zempsky WT. Clinical implications of unmanaged needle-insertion pain and distress in children. Pediatrics. 2008;122:S130-S133.
22. MacLean S, Obispo J, Young KD. The gap between pediatric emergency department procedural pain management treatments available and actual practice. Pediatr Emerg Care. 2007;23:87-93.
23. FDA Public Health Advisory. Potential hazards of skin products containing numbing ingredients for relieving pain from mammography and other medical tests and conditions.
www.fda.gov/cder/drug/ 2006;102:411-414.
24. Young KD. What's new in topical anesthesia. Clin Ped Emerg Med. 2007;8:232-239.
25. Ramsook C, Kozinetz CA, Moro-Sutherland D. Efficacy of ethyl chloride as a local anesthetic for venipuncture and intravenous cannula insertion in a pediatric emergency department. Pediatr Emerg Care. 2001;17:341-343.
26. Baxter AL. Topical anesthetics in children.
www.medscape.com. Accessed January 25, 2009.
27. Lysakowski C, Dumont L, Tramèr MR, Tassonyi E. A needle-free jet-injection system with lidocaine for peripheral intravenous cannula insertion: a randomized controlled trial with cost-effectiveness analysis. Anesth Analg. 2003;96:215-219.
To comment on this article, contact rdavidson@jobson.com.





