US Pharm. 2010;35(5):HS-2-HS-7.
Effective management of postoperative pain is a primary concern for health care practitioners and patients undergoing surgical procedures. Undertreatment of postoperative pain can result in negative physiologic and psychologic outcomes for the patient. It may then transform into chronic pain, impacting the financial burden on the health care system and leading to a significant decrease in the patient’s quality of life. There are a limited number of guidelines available to assist practitioners in the treatment of acute pain, with even fewer available for treatment of postoperative pain. Most guidelines focus on pain management by anesthesiologists in the perioperative setting.
This article addresses the management of acute postoperative pain with a focus on patient assessment, commonly used medications, routes of administration, and patient follow-up.
Types of Pain
The etiology of pain is quite complex. The International Association for the Study of Pain defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.1 Practitioners need to appreciate that pain is a condition that is extremely subjective for the patient. Therefore, therapy must be individualized to the patient and the patient’s current situation.
The experience of pain is mediated through a number of pathways in the body involving a variety of chemoreceptors and neuronal stimuli. Pain can be classified as either acute or chronic, depending on its onset and how it responds to treatment. Acute pain usually occurs from a known injury or trauma and responds well to treatment with medications, whereas chronic pain is long-standing and does not resolve quickly, even when treated. The management of chronic pain is difficult, with patients often having to try several medications before they achieve adequate pain relief. Nociceptive pain (normal pain in response to injury of the body) occurs usually as a result of tissue injury and is most often the type of acute pain experienced after surgery.2,3 Studies suggest that if acute pain is not treated appropriately it may transform into a chronic pain–type syndrome.4 There also may be neuronal damage as a result of surgery, which may result in chronic neuropathic pain (caused by damage to or a malfunction within the nervous system). Untreated postoperative pain may also be associated with delayed wound healing, which may complicate the postoperative course.5 The practitioner must be prepared to approach postsurgical pain treatment from a variety of angles, possibly trying a number of different medication classes to adequately treat the individual patient.
Patient Assessment
In order to begin to effectively treat postoperative pain, that pain must first be quantified in some manner. A patient’s self-reporting of pain intensity is the gold standard for pain assessment.2 When conducting patient assessment, the patient should be questioned as to the type of pain, the quality of the pain, what (if anything) seems to relieve the pain, the location of the pain, and pain severity.6 Patient assessment is done routinely after surgery to measure the success of pain management. There is also limited support for preoperative pain assessment since presurgical pain levels are thought to be a predictor of the amount of postsurgical pain that a patient may feel.7 When administering medication, it is recommended that the patient be assessed 30 minutes after administration of an IV dose of pain medication and 60 minutes after an oral dose to see if the medication was effective.6,8 There are numerous pain-measurement scales available for practitioners. Two of the more commonly used assessment tools are the numerical rating scale and visual analog scale. The numerical rating scale is primarily used in adult patients where they are asked to rate the intensity of pain on a scale from 0 to 10, with 0 being no pain and 10 being the most severe pain imaginable. The visual analog scale is a measurement instrument that asks the patient to place a mark on a continuous line that correlates with the severity of their pain. A score of 0 to 10 is then assigned to the patient’s mark depending on the location. In general, a score of 1-3 represents mild pain, 4-6 represents moderate pain, and 7 or greater is severe pain. It should be kept in mind, though, that how these scores correlate with the intensity of pain may vary slightly among pain-measurement assessment tools.
The type of assessment tool used may affect the accuracy of the response, depending on the type of patient.8 Practitioners must be able to identify and utilize the appropriate pain-scale assessment tool for that particular patient. Children, the elderly, and non-English- speaking patients may not understand certain standard pain scales and may benefit from the use of alternative pain scales such as the Faces pain scale (a series of faces depicting a patient experiencing varying degrees of pain). Practitioners must make sure that patients understand the pain scale and that they are able to correctly correlate the scores with their pain. Patients may also overreport or underreport their pain depending on the type of health practitioner administering the test. Patients may tend to be more honest about their true pain levels when nurses are asking about their pain and may underreport their pain levels when questioned by physicians.9 Certain preoperative variables or predictors, such as age, anxiety levels, and symptomatic depression, may also have an effect on the amount of pain that a patient will feel after surgery.10 Also, patients who have some degree of tolerance to opioid medications prior to surgery may require higher postoperative doses to adequately control their pain.11 Taking all of this into consideration only adds to the complexity of pain treatment and makes proper assessment that much more important. It is critical to understand that the patient’s level of pain is what he or she says it is and should always be treated as such.6
Medication Therapy Options
There are numerous medications that may be used for postoperative pain treatment, but these traditionally fall into a few select classes. The World Health Organization (WHO) developed a stepwise approach to chronic pain management, which is often applied to the management of acute pain. This is known as the WHO pain ladder.12 Opioids (synthetic opium derivatives) are used for the treatment of moderate-to-severe pain. (The term opiates refers to naturally occurring and semisynthetic opium agents.) Nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen is usually used for mild-to-moderate pain or as adjuvant therapy. There are oral and IV formulations of opioids and NSAIDs that are helpful in certain patient populations (e.g., patients who have undergone surgery). There are also numerous nonopioid analgesics such as N-methyl D-aspartate (NMDA) receptor antagonists (e.g., ketamine, amantadine), antidepressants (e.g., tricyclics, selective serotonin reuptake inhibitors), and antiepileptics (e.g., gabapentin, pregabalin), as well as agonist-antagonist combination therapy, which may have some benefit in certain situations. Although the type of agent prescribed needs to be individualized to the patient, there are certain general recommendations that should be applied when choosing the proper medication.
Opioids are widely used in the treatment of moderate-to-severe pain (pain scores 4 or greater). Opioids bind to the mu receptors in the central nervous system, resulting in analgesia. Common side effects include nausea and vomiting, constipation (exacerbated by increased periods of immobility that often follow surgical procedures), sedation, and pruritis. These adverse effects may be more pronounced in the elderly.6 Tolerance often develops to most of these side effects over time, with the main exception being constipation. A stimulant laxative should be prescribed when initiating opioids due to decreased peristalsis. Respiratory depression and hypotension are serious side effects of opioid therapy. Respiratory depression is defined as fewer than 10 breaths per minute, although specific hospital protocols may vary. The risk of severe respiratory depression is increased in the first 24 hours following surgery and with higher doses of opioids.13 An opioid antagonist, such as naloxone, should always be available in case of respiratory depression associated with overtreatment with an opioid medication. The recommended dose of naloxone for reversal of respiratory depression associated with overdose is 0.5 mL of naloxone 0.4 mg/mL added to 10 mL of normal saline, given by IV push every 2 minutes.6,14,15 The exact dose of naloxone should be titrated for each patient to minimize severe pain crises. Hypersensitivity reactions may also occur with opioids. It is important to note that if a hypersensitivity reaction occurs to an opioid, care should be taken not to rechallenge the patient with another medication in the same or similar class of medications, such as tramadol or tapentadol.
Tolerance may occur with opioids. However, it is not usually a concern when they are used for short-term acute pain treatment.16 Clinicians are often concerned with the addictive properties of opioids and may be hesitant to use them to treat postoperative pain. There is no evidence that the use of opioids in the postoperative setting increases the risk of addiction.6 Thus, pain should always be effectively treated. Undertreatment of postoperative pain can lead to greater suffering for the patient, an increase in postoperative admissions, and decreased quality of life.
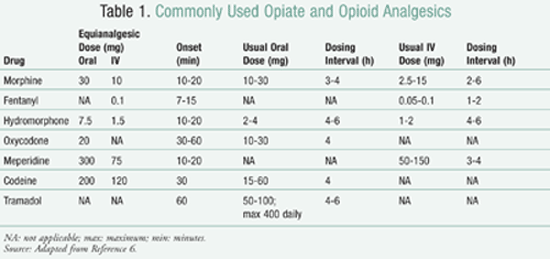
Interconversion between different agents within the opioid class is something that practitioners should be able to do, should a patient need to be switched between agents for any reason. TABLE 1 gives equianalgesic doses of commonly prescribed medications.
NSAIDs inhibit the enzyme cyclooxygenase (COX), thereby blocking the production of prostaglandins. Various prostaglandin subtypes are present throughout the body, including the vascular smooth muscle and the gastrointestinal (GI) tract. They are involved in a number of processes, including inflammation and a reduction in gastric acid secretion. Inhibition of the COX enzyme and, therefore, reduction in prostaglandin formation, results in anti-inflammatory responses. However, inhibition of the gastroprotective prostaglandin subtypes in the gut also increases the risk of gastric bleeding with these agents. NSAIDs are classified by their selectivity of the COX isoenzymes. Nonselective NSAIDs such as ibuprofen inhibit both COX-1 and COX-2 isoenzymes. Currently, there is only one COX-2 selective NSAID on the market, celecoxib, which has efficacy similar to the nonselective agents. NSAIDs are useful agents in mild-to -moderate cases of pain and have central and peripheral anti-inflammatory activity. Nonselective NSAIDs are associated with numerous side effects including an antiplatelet effect and an increased risk of bleeding. The risk of bleeding appears to be decreased when using a COX-2 agent.6 Postoperative bleeding risk should be a consideration depending on the type of surgery performed. Many studies have compared medications within the NSAID class and their ability to reduce pain experienced by patients. The general consensus in the recent literature is that COX-1 inhibitors are preferred over COX-2 inhibitors, especially in light of recent evidence of cardiovascular risk associated with COX-2 selective agents.17 It is important to note that nonselective agents have serious cardiovascular risks as well with the only exception being naproxen.6 Agents within this class have similar efficacy, and treatment should be individualized to the particular patient’s pain.
Due to the risk of gastrointestinal bleeding, NSAIDs might not always be the best choice depending on patient risk factors. Elderly patients, especially those who are dehydrated or are already on daily aspirin therapy, are at increased risk for GI bleeding. Pharmacists should be aware that there is also the potential for two key drug interactions between aspirin and NSAIDs. Not only are patients taking concomitant aspirin and an NSAID at a greater risk for GI bleeding, but some NSAIDs such as ibuprofen may negate the antiplatelet effects of low-dose aspirin, putting the patient at greater cardiovascular risk. Naproxen and celecoxib have not been shown to negate these antiplatelet effects and may be prescribed when patients are on low-dose aspirin. Chronic corticosteroid use is also associated with a higher risk of GI bleeds. If GI bleeding is a concern, agents such as misoprostol or proton pump inhibitors (PPIs) may be administered as a preventive measure.6
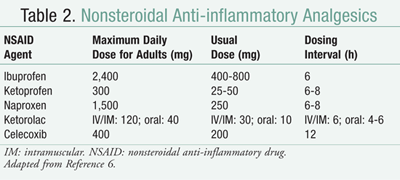
A major difference between opioids and NSAIDs is that unlike opioids, NSAIDs have a ceiling dose. There is a point when increasing the dose of NSAIDs does not result in additional analgesic benefits but significantly increases the risk of side effects. Each medication has a maximum dose that should not be exceeded. Some common NSAIDs and their associated maximum daily dose are listed in TABLE 2.
Acetaminophen is a centrally acting analgesic. Like NSAIDs, it is a good choice for mild-to-moderate cases of pain. However, unlike NSAIDs, acetaminophen lacks peripheral anti-inflammatory effects. Acetaminophen is a common ingredient in many nonprescription products and combination pain medications, so it is important to counsel the patient not to exceed 4,000 mg of acetaminophen daily from any and all sources. Also, alcohol consumption increases the risk of hepatotoxicity associated with this medication.18 Acetaminophen should be avoided in patients with a history of alcohol abuse or those with liver disease.
Tramadol is another option for moderate pain. It works at the mu receptor, in a similar manner to opioid analgesics, as well as at norepinephrine and serotonin receptors. It is generally associated with fewer opioid-like side effects. This agent should therefore be considered for use in patients who have intolerable side effects with opioids and for patients with chronic pain.6,14 Care should be taken not to exceed the maximum daily dose of 400 mg due to a significant risk of seizures in higher doses. Another newer available agent is tapentadol, which has a similar combined effect on the mu receptor and norepinephrine receptors. The recent approval of tapentadol increases the available options for medications with mechanisms of action that vary slightly from traditional opioid receptor agonism.
Combination therapy of an opioid and nonopioid is commonly utilized. Combining medications that work at different areas of the pain cascade or by differing mechanisms may reduce the amount of each medication required to achieve pain control, thereby reducing the potential for side effects. However, the combination of an opioid and nonopioid may hinder the dose titration of the opioid product due to the ceiling effect of the nonopioid. If using a combination product, care should be taken not to exceed the maximum daily dose of either medication in the combination. More recently, agonist-antagonist combinations have been studied as a means of achieving analgesia while minimizing tolerance and the occurrence of side effects. Studies are currently inconclusive as to the actual benefit of the addition of naloxone to epidural injections or oral therapy in reducing postoperative pain levels.6,16
Patient-controlled analgesia (PCA) is a method of administration in which either IV, subcutaneous, or epidural analgesia is administered in small doses by the patient himself or herself. Morphine and hydromorphone are two of the most common opioid products chosen for a PCA, although other opioids, such as fentanyl, may be used. PCA is associated with better pain scores upon patient assessment.19 However, it is also associated with the highest incidence of medication error.20 PCA should not be administered to patients with any cognitive impairment, which would limit their ability to properly use this technique of analgesia, or to any patients who are reluctant to take over control of their pain treatment.14 PCA is typically administered by use of a pump, which must be calibrated. Most pumps can be programmed to administer continuous basal infusions of medication, small boluses of medication, or both. There is a high rate of calculation errors associated with PCA drug dosing and pump set-up. Institutional PCA protocols should be in place if PCA is to be used safely and effectively.20 It is important to note that clinical boluses of medication may be given to the patient postoperatively by a health care professional until the patient is awake and able to self-administer the PCA dose.
Newer, Nontraditional Methods and Agents
When considering postsurgical pain, there is some debate as to whether preemptive pain control should be done, or whether medication administration should be done solely based on patient demand after surgery. Preemptive medication administration is the administration of medication prior to surgery in an attempt to reduce the amount of postoperative pain medication required by the patient. Preoperative use of gabapentin and pregabalin has been studied, and the results of studies on opioid consumption postoperatively are varied.21 More studies need to be done at this point. However, it is apparent that preoperative medication administration may have a positive effect on postoperative outcomes. The use of NMDA antagonists, such as ketamine, is currently being studied. N-methyl D-aspartate is a glutamate receptor in the CNS that is responsible for memory function and pain sensitization. It is believed that by blocking NMDA receptors, pain may be modulated and the severity of pain reduced. NMDA receptor antagonists are currently used in animals and humans as anesthetics. Though it is questionable as to whether the use of NMDA antagonists has an opioid-sparing effect, there may be some support that the use of NMDA antagonists may prove to be a viable alternative or adjunct therapy to traditional pain medications.8,22-25 More studies need to be done to assess the full benefit of NMDA antagonists in postoperative pain control.
Preoperative patient counseling and preparation as to expectations regarding surgery and the associated postsurgical pain may have some benefit. Patients adequately prepared with what to expect have been shown to have lower postoperative pain scores. Therefore, this should be tied into patient assessment measures done prior to surgery.14
Current Recommendations
Intravenous medications, administered either to the patient or by the patient with PCA, allow for a quicker onset of treatment and better control over patient symptoms. Oral medications typically have a longer onset of action, which extends the length of time that a patient’s pain could go untreated. The benefit of oral medications is that they can easily be continued when the patient goes home. If oral medications are chosen for acute postoperative pain, shorter-acting agents with a quicker onset of action used “as needed” by the patient are preferable.14
Once the decision is made to switch the patient to oral therapy for discharge, the appropriate dose and type of medication can be decided upon based on the type of therapy the patient responded to as an inpatient. Patients should be counseled prior to discharge about medication use and side effects. They should be made aware of all follow-up appointments. They should be counseled that if they are not achieving adequate pain control with the medication, the prescriber should be notified so that therapy can be adjusted accordingly.14
There are many options when it comes to the management of postoperative pain. Accurate patient assessment is one of the most important factors in choosing the most effective drug therapy in each given case. Studies continue to be done on new modes of therapy that may serve to expand available treatment options in the future. The pharmacist can play a vital role in the appropriate treatment of pain and in patient assessment and counseling. Each patient is unique in his or her perception of pain, and this uniqueness affords the clinician many opportunities for involvement in the treatment of the patient and in reducing complications and unneeded patient discomfort after surgery.
REFERENCES
1. International Association for the Study of Pain. www.iasp-pain.org/AM/Template.cfm?Section=General_Resource_Links&
Template=/CM/HTMLDisplay.cfm&ContentID=3058#Pain. Accessed November 24, 2009.
2. Fine PG, Portenoy RK. A Clinical Guide to Opioid Analgesia. New York, NY: McGraw-Hill. www.stoppain.org/pcd/content/forpros/opioidbook.asp. Accessed November 23, 2009.
3. Merck Manual. Pain. www.merck.com/mmpe/sec16/ch209/ch209a.html?qt=pain&alt=sh. Accessed November 24, 2009.
4. Kehlet H, Jensen TS, Woolf CJ. Persistent post-surgical pain: risk factors and prevention. Lancet. 2006;367:1618-1625.
5. Joshi G, Ogunnaike B. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiology Clin N Am. 2005;23:21-36.
6. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Chronic Cancer Pain: A Concise Guide to Medical Practice. 5th ed. Skokie, IL: APS; 2003.
7. Kalkmann CJ, Visser K, Moen J, et al. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415-423.
8. Institute for Clinical Systems Improvement. Health Care Guideline: Assessment and Management of Acute Pain. 6th ed. Bloomington, MN: ICSI; 2008.
9. Sills E, Genton M, Walsh A, Wehbe S. Who’s asking? Patients may under-report postoperative pain scores to nurses (or over-report to surgeons) following surgery of the female reproductive tract. Arch Gynecol Obstet. 2009;279:771-774.
10. Caumo W, Schmidt AP, Schneider CN, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265-1271.
11. Patanwala A, Jarzyna D, Miller M, Erstad B. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naïve patients after total knee arthroplasty. Pharmacotherapy. 2008;28:1453-1460.
12. World Health Organization. www.who.int/cancer/palliative/painladder/en/. Accessed March 20, 2010.
13. Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190:752-756.
14. Veterans Health Administration. Clinical Practice Guideline for the Management of Postoperative Pain. Version 1.2. July 2001. www.healthquality.va.gov/pop/pop_fulltext.pdf.
15. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004;100:1573-81.
16. Collett BJ. Opioid tolerance: the clinical perspective. Brit J Anaesth. 1998;81:58-68.
17. Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59:117-133.
18. U.S. Food and Drug Administration. Acetaminophen Information. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.html. Accessed December 17, 2009.
19. Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain: a quantitative systematic review. Acta Anaesthesiol Scand. 2001;45:795-804.
20. Hicks RW, Sikirica V, Nelson W, et al. Medication errors involving patient-controlled analgesia. Am J Health-Syst Pharm. 2008;65:429-440.
21. Jokela R, Ahonen J, Tallgran M, et al. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134:106-112.
22. Nesher N, Serovian I, Merouani N, et al. Ketamine spares morphine consumption after transthoracic lung and heart surgery without adverse hemodynamic effects. Pharmacol Res. 2008;58:38-44.
23. Jensen LL, Handberg G, Helbo-Hansen HS, et al. No morphine sparing effect of ketamine added to morphine for patient-controlled intravenous analgesia after uterine artery embolization. Acta Anaesthesiol Scand. 2008;52:479-486.
24. Cambell-Fleming J, Williams A. The use of ketamine as adjuvant therapy to control severe pain. Clin J Oncol Nurs. 2008;12:102-107.
25. Snijdelaar DG, Koren G, Katz J. Effects of perioperative oral amantadine on postoperative pain and morphine consumption in patients after radical prostatectomy. Anesthesiology. 2004; 100:134-41.
To comment on this article, contact rdavidson@uspharmacist.com.





