US Pharm.
2007;32(10):52-56.
Despite improvements in the field of dentistry,
dental decay remains the number one illness among children, surpassing asthma.
1 Dental professionals are striving to reach young children, especially
those at high risk for dental decay, so that preventive programs can be
implemented and routine oral health care can be established early. Current
recommendations by the American Dental Association (ADA) and the American
Academy of Pediatric Dentistry (AAPD) state that all children should have
their first dental visit by age 1 year or within six months after the first
tooth erupts. More and more dentists are implementing infant oral health care
and anticipatory guidance programs into their practices.2 The goal
is to reach parents early and give them advice in hopes of preventing dental
decay in children. Anticipatory guidance counseling that occurs in the dental
office frequently includes counseling parents about their child's diet,
fluoride exposure, home care, oral habits, dental injury prevention, and risk
of caries.3
One of the most effective agents used today
to combat tooth decay is fluoride. Fluoride is a proven mainstay in preventive
oral health care, and children at risk of developing dental decay should be
evaluated for systemic and topical fluoride exposure by their oral health care
provider.4 Children may be exposed to a variety of fluoride
sources, and it is important to ensure that they are not receiving excessive
or insufficient amounts of fluoride. If a child is not receiving adequate
fluoride, a prescription or OTC supplement may be prescribed. For optimal
prevention of decay for the developing and erupted teeth, it is recommended
that children be exposed to fluoride at age six months. Supplementation in the
form of tablets, mouth rinses, pastes, or gels is generally continued until
age 16. Pharmacists are in the ideal position to counsel parents on their
child's fluoride status, as they may be filling fluoride prescriptions
frequently. They may also recommend age-appropriate toothpastes and mouth
rinses containing fluoride from the readily available OTC products.
Pharmacology
Fluoride is thought to be
effective in combating dental decay both systemically and topically. When
ingested, almost 100% of fluoride is absorbed in the gastrointestinal (GI)
tract, approximately 90% in the stomach.5 Fluoride absorption is
inhibited by calcium, magnesium, and iron and is excreted primarily through
the kidneys and, to a lesser extent, the sweat glands, the GI tract, and
breast milk. Once ingested, the fluoride has a systemic affect on teeth before
they erupt, incorporating into the matrix of developing teeth to increase the
mineralization content and decrease the solubility of enamel.6
Topical effects of fluoride come from applying it directly onto teeth already
erupted, thereby promoting remineralization, increasing tooth resistance to
acid dissolution, and inhibiting cariogenic activity of bacteria in the mouth.
Drinking fluoridated water and ingesting fluoride prescription drops,
lozenges, or tablets are all ways of receiving adequate systemic fluoride with
some simultaneous topical effects, while using toothpastes, gels, and mouth
rinses containing fluoride exerts topical effects. It is speculated that
systemic fluoride supplements may decrease caries rates by 60% and that
topical application may decrease caries activity by up to 40%.5
Fluoride Overdosage
Symptoms of acute fluoride
overdosage range from excessive salivation, nausea, vomiting, abdominal pain,
and diarrhea to central nervous system irritability, paresthesias, tetany,
convulsions, and respiratory and cardiac failure. Laboratory findings include
hypocalcemia, hypoglycemia, and delayed hyperkalemia. A dose of 70 to 140
mg/kg is considered lethal.5 In cases of acute fluoride overdosage,
patients should call their physician or local poison control center or go to
their local emergency room. Management is aimed first at preventing the
absorption of fluoride by administering calcium, which acts as a binding
agent, in the form of milk, milk of magnesia, or calcium carbonate.7
Adjunctive treatment includes normalizing pH and electrocytes and general
toxicity support. Extreme fluoride toxicity cases may require dialysis or
hemoperfusion to remove excess fluoride from the body.7
Children suffering from chronic fluoride
overdosage experience dental fluorosis, exhibited by pitting or discoloration
of enamel, and osseous changes such as elevated bone density.8-10
To assess chronic fluoride overdosage and dental fluorosis, the oral health
care provider must take a detailed history of past and pres ent fluoride
intake--considering all the sources of fluor ide the child has been
exposed to, including environmental fluoride, OTC products, and prescription
supplements--and adjust the fluoride exposure to recommended levels.
Fluoride Exposure Through Drinking Water
The most common and easy way for
a child to receive systemic fluoride is through drinking water. Throughout the
country, most municipal water treatment centers add fluoride to the community
water during the water treatment process. It is thought that the fluoride
level must be at least 1 part per million (ppm) to be efficacious in
preventing dental decay.11 Children who live in fluoridated
communities and drink the tap water in their homes on a daily basis have added
protection against tooth decay. Parents can also be advised to use it while
cooking at home or mixing formula for infants.
Children living in rural areas in homes with
water supplied by a private or public groundwater well, however, may need
their water tested to evaluate the fluoride concentration present.12
Home water-testing kits are readily available from municipal water facilities
or state environmental protection agencies and can be performed at the request
of the child's dentist or pediatrician. If the fluoride content is revealed to
be less than 1 ppm, then a systemic fluoride prescription is recommended and
can be prescribed by the provider. If these children attend school or daycare
in a fluoridated community, they are considered protected if they are drinking
the water while at school on weekdays. They may be advised to drink
fluoridated bottled water on the weekends and may not require additional
systemic fluoride supplementation. Alternatively, these children may also be
prescribed systemic fluoride supplements to take on weekends only.
For children living in fluoridated-water
communities, drinking tap water that is filtered through the refrigerator or
other commercial filtering products is recommended; fluoride is not removed
through most filtering processes.
Bottled Water Containing Fluoride
A relatively new product found on
the shelves of most grocery stores and pharmacies today is bottled water
containing fluoride. It should be noted that not all bottled water contains
fluoride; the labeling must state that fluoride has been added. This is
usually done on colorful labels marketed toward children and infants. The
fluoride is added with the express purpose of preventing dental decay and is
now manufactured by most major bottled-water brands. Dental professionals are
now recommending that parents of children living in communities without
fluoridated water purchase this bottled water and have their child drink it
daily. Theoretically, consuming this bottled water daily will have the same
protective benefit as drinking the home water in a fluoridated community thus
avoiding the need for prescription supplements.
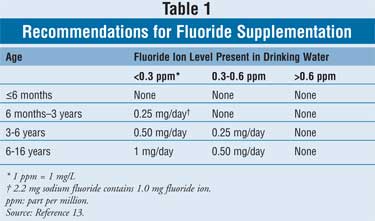
Prescription Systemic Fluoride
Supplemental fluoride may be
prescribed in the form of drops, lozenges, or chewable tablets. The dosing for
fluoride is dependent on the child's age and the home water fluoride content (
Table 1).13 Prescription fluoride products are dosed as "mg of
fluoride ion." However, pharmacists may see the health care provider writing
the prescription stating the fluoride dosage as "mg sodium fluoride."
Dispensing the correct dosage can be accomplished using the simple conversion
factor of 2.2 mg sodium fluoride equaling 1.0 mg fluoride ion.
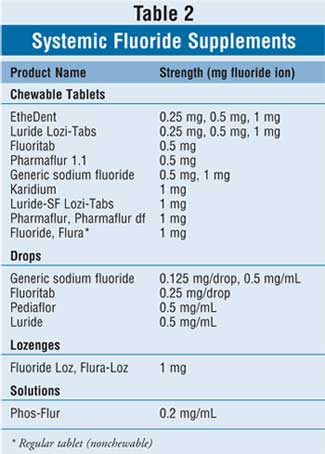
The myriad of available oral systemic
fluoride supplements is listed in Table 2. Supplemental fluoride is
available in lozenges as 1 mg fluoride ion (equal to 2.2 mg sodium fluoride).
Tablets are typically manufactured as 0.25, 0.5, and 1.0 mg fluoride ion
(equal to 0.55, 1.1, and 2.2 mg sodium fluoride, respectively). Children older
than 4 years should be instructed to suck on one lozenge or chew one tablet
for one to two minutes before swallowing each night at bedtime. Children aged
4 years or younger should be prescribed liquid fluoride drops. Dosed as 0.125,
0.25, and 0.5 mg fluoride ion (equal to 0.275, 0.55, and 1.1 mg sodium
fluoride, respectively), the drops should be given once daily much like a
multivitamin.
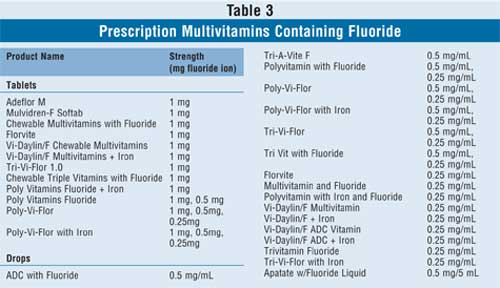
In addition to the products that contain
fluoride only, there are also many prescription multivitamin drops and tablet
preparations available that consist of a variety of vitamins in different
combinations, including vitamins A, D, E, C, and B; folic acid; iron; and
fluoride (Table 3). The health care provider may prescribe these
products if the child is in need of multivitamin and fluoride supplementation
concomitantly.
Topical Fluoride
Fluoridated toothpaste is the
most common source of topical fluoride and should be used twice daily when
brushing. Children younger than 2 years should not use fluoridated toothpaste
to avoid possible toxicity.14 Parents can brush with a toothbrush
wet with water or they may choose to use toothpaste without fluoride. Children
older than 2 years should use a small smear of fluoridated toothpaste no
larger than the size of a green pea on their brush; toothpaste marketed for
adults or children can be used, as long as it bears the ADA Seal of Acceptance
(Figure 1).

Mouth rinses containing high-strength
fluoride may also be implemented, particularly in high-risk children; commonly
used products may be found in Table 4. OTC mouth rinses that contain
fluoride must bear the ADA Seal of Acceptance and may be used once daily at
bedtime after brushing and flossing in children older than 4 years who can
expectorate reliably. Similarly, there are a few fluoridated mouth rinses
available by prescription only. Fluoridated mouth rinses, both OTC and
prescription, are unsafe in children who cannot expectorate reliably due to
toxicity issues.15 Mouth rinses have optimal effect if patients
rinse for one minute, then expectorate; nothing else should be taken by mouth
for at least 30 minutes afterward.
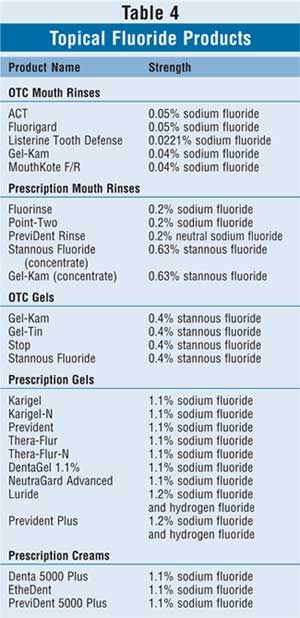
Fluoride-containing gels and creams are
available OTC or by prescription and are reserved for children with high
cavities rates and children undergoing active orthodontic treatment who may
have appliances in their mouths such as retainers or braces (Table 4).
Best used at bedtime immediately after brushing and flossing, fluoride gels
and creams should be brushed on for approximately one minute before fully
expectorating. Further, nothing should be taken by mouth for at least 30
minutes after the fluoride exposure to ensure optimal efficacy.
The Pharmacist's Role
While fluoride is a very safe and
effective drug to prevent cavities, it is crucial to understand its toxicity
limits to prevent harm to children who have access to fluoride in the home in
the form of toothpastes, mouth rinses, gels, creams, or prescriptions. With
such a variety of ways to deliver fluoride and help prevent tooth decay, the
pharmacist can play in integral role in counseling parents to ensure that
their children are receiving adequate fluoride. It is important that the
pharmacist counsel parents on the proper use of fluoridated water and OTC and
prescription products to secure the greatest benefits of fluoride while
avoiding overdosage. The ultimate goal is to have a generation of children
with the right amount of fluoride exposure so that they may have little to no
caries activity, leading to happier dental visits for everyone.
References
1. Centers for Disease Control and Prevention. Progress reviews for healthy people 2000: oral health. Briefing book materials: disparity charts. December 1999. Available at: www.cdc.gov/nchs/about/otheract/hp2000/oralhealth/oralhealth.htm.
2. Ramos-Gomez FJ. Clinical considerations for an early infant oral health care program. Compend Contin Educ Dent. 2005;26:17-23.
3. Lee JY, Bouwens TJ, et al. Examining the cost-effectiveness of early dental visits. Pediatr Dent. 2006;28:102-105.
4. American Academy of Pediatric Dentistry Councils on Clinical and Scientific Affairs. Reference Manual. Pediatr Dent . 2007;28:29-30.
5. Drug Facts and Comparisons 4.0. Fluoride. Available at: www.efactsweb.com. Accessed June 20, 2007.
6. Limeback H. A re-examination of the pre-eruptive and post-eruptive mechanism of the anti-caries effects of fluoride: is there any anti-caries benefit from swallowing fluoride? Community Dent Oral Epidemiol. 1999;27:62-71.
7. McIvorME. Acute fluoride toxicity: pathophysiology and management. Drug Saf. 1990;5:79-85.
8. Levy SM, Warren JJ, Broffitt B, Kanellis MJ. Associations between dental fluorosis of the permanent and primary dentitions. J Public Health Dent. 2006;66:180-185.
9. Hallanger Johnson JE, Kearns AE, et al. Fluoride-related bone disease associated with habitual tea consumption. Mayo Clin Proc. 2007;82:719-724.
10. Tamer MN, Kale Koroglu B, et al. Osteosclerosis due to endemic fluorosis. Sci Total Environ. 2007;373:43-48.
11. Committee on Fluoride in Drinking Water, National Research Council. Fluoride in Drinking Water: A Scientific Review of EPA's Standards. The National Academies Press; 2006.
12. Horowitz HS. The role of dietary fluoride supplements in caries prevention. J Public Health Dent. 1999;59:205-210.
13. American Dental Association. Fluoride and fluoridation. Available at: www.ada.org/public/topics/fluoride. Accessed June 20, 2007.
14. Adair SM, Piscitelli WP, McKnight-Hanes C. Comparison of the use of a child and adult dentifrice by a sample of preschool children. Pediatr Dent. 1997;19:99-103.
15. Adair SM. The role of fluoride mouthrinses in
the control of dental caries: a brief review. Pediatr Dent.
1998;20:101-104.
To comment on this article, contact
editor@uspharmacist.com.





