US Pharm. 2012;37(3):HS-12-HS-15.
The World Health Organization estimates that more than 150 million cases of pneumonia occur each year in children aged <5 years.1 Pneumonia is a leading cause of morbidity and mortality in this population, resulting in approximately 1.4 million deaths annually—more than AIDS, malaria, and tuberculosis combined.2 While pediatric pneumonia is more prevalent and deadly in the developing world, it is common in Europe and North America, occurring at a rate of 4 cases per 100 preschool-aged children, 2 cases per 100 children aged 5 to 9 years, and 1 case per 100 children aged 9 to 15 years.1-3 The CDC reported that, from 2008 to 2009, the rate of infant mortality from pneumonia in the United States was 5.1 deaths per 100,000.4
Practice guidelines for the management of adults with community-acquired pneumonia (CAP) have been demonstrated to reduce associated morbidity and mortality.5,6 In an attempt to extrapolate these positive outcomes to the pediatric population, the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) released new (August 2011) guidelines for the management of CAP in infants and children aged >3 months.7 This review is intended to educate pharmacists and pharmacy technicians about the proper management of CAP in immunocompetent children according to these guidelines.
Etiology and Risk Factors
Determining the cause of pneumonia in children is often difficult. Sputum from the lower respiratory tract can rarely be obtained from children. As with adults, culturing the upper respiratory tract is of little value, as the normal flora in this area may not be responsible for the pneumonia.8 Several investigations have explored the microbial etiology of CAP.9-12 These studies vary considerably in their etiologic findings. The use of different evaluative laboratory tests between studies poses a challenge in comparing the causes of pneumonia. Despite these variations, it is widely accepted that the most prominent pathogens responsible for CAP in children are viral and bacterial in nature.7,8 It is important to note that children often present with combined infections of multiple viruses, bacteria, or both.7,8
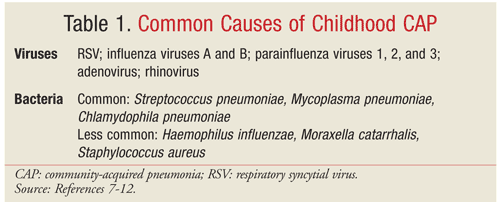
Pediatric CAP exhibits age-related causation because children may be exposed to different pathogens in various age-related settings (home, day care, school) and because, with the development of immunity, children become less likely to acquire certain infections and more likely to develop others.7,8 In children aged 3 months to 5 years, viruses are the most common cause of pneumonia, but Streptococcus pneumoniae and atypical bacteria—particularly Mycoplasma pneumoniae and Chlamydophila pneumoniae—are also common.7 In children aged >5 years, S pneumoniae, M pneumoniae, and C pneumoniae are the most important causes of pneumonia.7 The microorganisms most frequently associated with pneumonia in children are listed in TABLE 1.7-12
There are several factors that may increase a child’s risk of acquiring CAP. Immunologic disorders, hematologic disorders, cardiac conditions, and chronic pulmonary conditions are considered significant risk factors for pneumonia.7 Other factors include preexisting illnesses such as HIV infection and measles.2 In addition, malnourished children and infants who are not exclusively breastfed are more likely to have a weakened immune system, which increases their risk of acquiring pneumonia.2 Finally, environmental factors, including air pollution, living in a crowded home, and parental smoking, heighten a child’s risk of infection.2
Pathophysiology
Pneumonia results from the proliferation of microbial pathogens and the host’s response to the offending microorganisms at the alveolar level of the lower respiratory tract.13 Microorganisms may gain access to the lower respiratory tract through four different pathways: inhalation of contaminated droplets, aspiration of oropharyngeal or gastrointestinal contents, hematogenous spread, and progressive extension from a contiguous site of infection.13 Pneumonia occurs after the host’s immune and nonimmune defense systems are breached and manifests when the capacity of alveolar macrophages to ingest, kill, and clear microorganisms is exceeded by the number of pathogens.13
Clinical Presentation
No single sign or symptom is pathognomonic of pneumonia in children. While the combination of fever and cough is suggestive of pneumonia, the clinical presentation may be subtle or misleading.14 Infants may present with a toxic appearance and nonspecific symptoms including poor feeding, hypotonia, floppiness, lethargy, and apnea.14 Older children are more likely to present with typical signs and symptoms of pneumonia.14 The strongest predictors of pneumonia in children are fever, cough, cyanosis, nasal flaring, retractions, rales, tachypnea, and reduced breath sounds.14
Treatment and Prevention
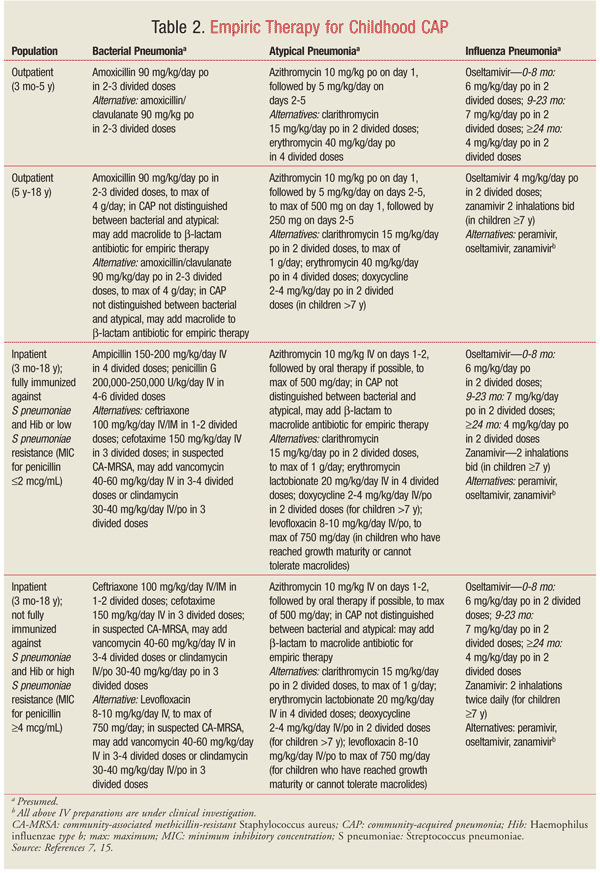
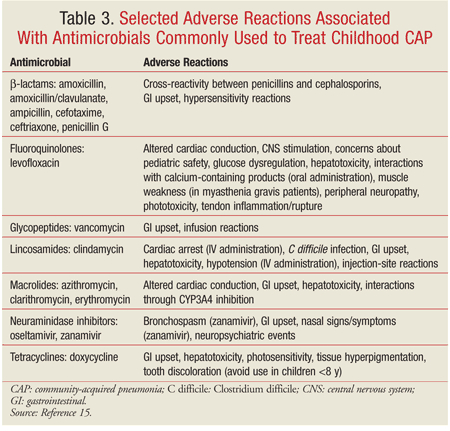
Empiric therapy for CAP in children should be directed at the most likely pathogens. Since pneumonia in infants and young children is usually caused by viruses, antibiotic therapy is not always necessary in this population. TABLE 2 summarizes the empiric treatment of CAP in children based on the 2011 PIDS/IDSA guidelines.7,15 Duration of therapy is usually 10 days for bacterial pneumonia and 5 days for influenza pneumonia.7 Some adverse reactions associated with antimicrobials frequently used to treat pediatric CAP are highlighted in TABLE 3.15
Three of the most common causes of pneumonia in children can be prevented with the use of currently licensed vaccines. Globally, S pneumoniae and Haemophilus influenzae type b (Hib) account for approximately one-half of deaths from pneumonia in children aged <5 years.16 Routine immunization against these pathogens has greatly reduced the incidence and severity of these types of pneumonia in the developed world. The CDC reported that, following the introduction of the heptavalent pneumococcal conjugate vaccine in 2000, severe pneumococcal disease dropped by almost 80% in children aged <5 years.17 In 2010, a 13-valent pneumococcal conjugate vaccine was introduced to further expand coverage to include serotype 19A, the most common serotype, which is frequently resistant to antibiotics.18 The CDC also noted that the incidence of Hib disease declined by 99% in children aged <5 years following the introduction of the Hib conjugate vaccine in 1990.19 There is evidence that vaccinating children against influenza reduces the incidence of influenza-related illnesses and complications, particularly bacterial pneumonia caused by S pneumoniae and S aureus.20 The vaccines commonly recommended for preventing childhood pneumonia, along with their schedules according to the CDC Advisory Committee on Immunization Practices, are listed in TABLE 4.21,22

The Pharmacist’s Role
Pharmacists should actively engage with other clinicians to recommend the most appropriate antimicrobial regimen for the treatment of a specific child with pneumonia, including dosing and therapy duration. Pharmacists are well trained to assess patients for the efficacy and safety of antimicrobial regimens, including monitoring children for adverse reactions and interactions. Pharmacists must educate health care professionals and patients about the judicious and appropriate use of antimicrobials to limit the spread of resistance, reduce side effects, and control costs. Finally, pharmacists play a pivotal role in preventing CAP by serving as immunization advocates, facilitators, and administrators, where applicable.
REFERENCES
1. Rudan I, Tomaskovic L, Boschi-Pinto C, et al. Global estimate of
the incidence of clinical pneumonia among children under five years of
age. Bull World Health Organ. 2004;82:895-903.
2. World Health Organization. Pneumonia. Fact Sheet No. 331. October
2011. www.who.int/mediacentre/factsheets/fs331/en/index.html. Accessed
November 19, 2011.
3. Denny FW, Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986;108:635-646.4. Kochanek KD, Xu J, Murphy SL, et al. Deaths: preliminary data for
2009. National Vital Statistics Reports, vol. 59, no. 4. Hyattsville,
MD: National Center for Health Statistics; 2011.
5. Dean NC, Bateman KA, Donnelly SM, et al. Improved clinical
outcomes with utilization of a community-acquired pneumonia guideline. Chest. 2006;130:794-799.
6. McCabe C, Kirchner C, Zhang H, et al. Guideline-concordant therapy
and reduced mortality and length of stay in adults with
community-acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169:1525-1531.
7. Bradley JS, Byington CL, Shah SS, et al. The management of
community-acquired pneumonia in infants and children older than 3 months
of age: clinical practice guidelines by the Pediatric Infectious
Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
8. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
9. Cevey-Macherel M, Galetto-Lacour A, Gervaix A, et al. Etiology of
community-acquired pneumonia in hospitalized children based on WHO
clinical guidelines. Eur J Pediatr. 2009;168:1429-1436.
10. Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical
characteristics of community-acquired pneumonia in hospitalized
children. Pediatrics. 2004;113:701-707.
11. Tsolia MN, Psarras S, Bossios A, et al. Etiology of
community-acquired pneumonia in hospitalized school-age children:
evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39:681-686.
12. Esposito S, Bosis S, Cavagna R, et al. Characteristics of Streptococcus pneumoniae and atypical bacterial infections in children 2-5 years of age with community-acquired pneumonia. Clin Infect Dis. 2002;35:1345-1352.
13. Mandell LA, Wunderink R. Pneumonia. In: Kasper DL, Fauci AS, eds. Harrison’s Infectious Diseases. New York, NY: McGraw-Hill Medical; 2010:188-201.
14. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Physician. 2004;70:899-908.
15. Lexi-Comp Online [online database]. Hudson, OH: Lexi-Comp, Inc; 2011. www.lexi.com. Accessed November 21, 2011.
16. Announcement: World Pneumonia Day—November 2, 2009. MMWR. 2009;58:1184.
17. CDC. Pneumococcal conjugate vaccine: what you need to know.
www.cdc.gov/vaccines/pubs/vis/downloads/vis-pcv.pdf. Accessed November
20, 2011.
18. CDC. PCV13 (pneumococcal conjugate) vaccine for providers.
www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-hcp-faqs.htm. Accessed
November 20, 2011.
19. CDC. Haemophilus influenzae serotype b (Hib) disease. www.cdc.gov/ncidod/dbmd/diseaseinfo/haeminfluserob_t.htm. Accessed November 20, 2011.
20. Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of
influenza with vaccines: recommendations of the Advisory Committee on
Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1-62.
21. CDC. Recommended immunization schedule for persons aged 0 through
6 years—United States, 2012.
www.cdc.gov/vaccines/recs/schedules/downloads/child/0-6yrs-schedule-pr.pdf.
Accessed February 7, 2012.
22. CDC. Recommended immunization schedule for persons aged 7 through
18 years—United States, 2012.
www.cdc.gov/vaccines/recs/schedules/downloads/child/7-18yrs-schedule-pr.pdf.
Accessed February 7, 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





