US Pharm. 2009;34(11):26-39.
Uncertainty is high when it comes to selecting the appropriate antidepressant for patients diagnosed with major depressive disorder (MDD), not only because studies have reported no differences in efficacy between agents, but also because only 11% to 30% of patients will reach remission with initial treatment, even after a year.1,2 This consequently has led clinicians to practice in a trial-and-error fashion to treat depression.3 Furthermore, the last major revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) was produced in 2000.4 In 2005, a guideline watch was published to review important safety concerns that had emerged about some agents, such as nefazodone, as well as to review two new antidepressants approved that year, escitalopram and duloxetine.5 Revision and update of the DSM-IV-TR (DSM-V manuscript) is not due until May 2012. Therefore, there is need for an up-to-date review to assist clinicians in deciding on the appropriate agents to treat individual patients.
The 2007 Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study attempted to develop and evaluate feasible treatment strategies to improve clinical outcomes for patients with treatment-resistant depression who were identified with a current major depressive episode.6 Specifically, STAR*D aimed to determine which of several treatments is the most effective “next step” for patients who do not reach remission with an initial or subsequent treatment or who cannot tolerate the treatment. The overall results of this study demonstrated that pharmacologic differences between psychotropic medications did not translate into substantial clinical differences, although tolerability differed.6
The purpose of this article is to review treatment evidence available in the literature in order to provide a quick reference that will help clinicians decide on the appropriate agent, taking into consideration adverse effects, drug interactions, and medication safety, as well as patient characteristics.
What Is Depression?
Depression can be a chronic or recurrent mental disorder that presents with several symptoms such as depressed mood, loss of interest or pleasure, feelings of guilt, disturbed sleep or appetite, low energy, and difficulty thinking.7 Depression can lead to substantial impairment in an individual’s ability to take care of everyday responsibilities. Depression can also lead to suicide, a tragedy accounting for the loss of about 850,000 lives worldwide every year.7
Prevalence and At-Risk Populations
There are an estimated 121 million people worldwide affected with depression.7 In 2000, depression was the fourth leading contributor to the global burden of disease among all diseases, and by 2020 it is anticipated that it will rise to the number-two leading contributor to the global burden of disease, second only to heart disease.7
Populations at higher risk for developing depression include women, people between the ages of 24 and 45 years, and those with first-degree relatives with depression. Women are at increased risk for depression until their 50s, and their lifetime risk is two times greater than men’s. People between the ages of 24 and 45 years experience the highest rate of depression. Finally, first-degree relatives of depressed patients are 1.5 to 3 times more likely to experience depression than others.4,8
Pathophysiology and Pharmacotherapy Rationale
Biological and psychosocial causes have been hypothesized in an attempt to describe the pathophysiology of depression. Pharmacologic agents will target the biological causes linked to dysregulation in the neurotransmitters. This dysregulation is often described as a deficiency in brain neurotransmitter levels. Norepinephrine, serotonin, and dopamine levels may be decreased in patients with depression, thus associating a decreased amount of neurotransmitters with the disorder.8 The pharmacotherapy rationale has been to boost these deficiencies by blocking the reuptake or preventing enzymatic metabolism of neurotransmitters via antidepressants. Overall, these mechanisms aim at increasing the level of neurotransmitters either by forcing the neuron to fire more often and produce more neurotransmitters or by preventing degradation of the neurotransmitter itself.
Pharmacologic Agents
Several classes of agents are currently available to treat depression. They include selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and serotonin-norepinephrine reuptake inhibitors (SNRIs), among others.
Antidepressants have been associated with an increased risk of suicidal thinking and suicidality in children, adolescents, and young adults in short-term placebo controlled studies of MDD. Anyone considering the use of any antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24 years, and there was a reduction in risk with antidepressants compared to placebo in adults aged 65 years and older. Depression and other psychiatric disorders are themselves associated with increased risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior.9
Selective Serotonin Reuptake Inhibitors4,10: SSRIs are considered first-line agents when it comes to treating patients with depression. These drugs include citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline (TABLE 1). The major adverse effects of this class include nausea, vomiting, and diarrhea, which are dose-dependent effects and tend to dissipate after the first weeks of treatment. In some patients, SSRIs can cause agitation and sleep disturbances, which will also dissipate with time. Sexual dysfunction is a side effect present with all antidepressants, but seems to be most common with SSRIs.
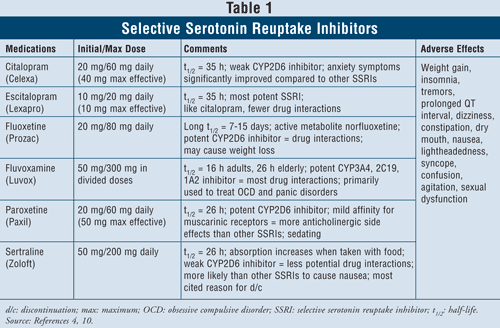
Serotonin syndrome (i.e., abdominal pain, diarrhea, sweating, mental status change, renal failure, cardiovascular shock, and possibly death) is a rare adverse effect of SSRIs. Serotonin syndrome can occur with an increase in SSRI dose or by taking SSRIs with herbals such as St. John’s wort or with illicit drugs. Finally, combining SSRIs with MAOIs can also lead to lethal drug interactions with development of serotonin syndrome. When clinicians feel the need to switch from one class of agent to the other, it is suggested that at least five half-lives elapse between the time the SSRI is stopped and the MAOI is started.4 Of all agents in the class, fluvoxa mine has the highest rate of drug interactions, since it inhibits several hepatic enzymes such as CYP450 1A2, 2C19, 2C9, 2D6, and 3A4. Fluoxetine follows fluvoxamine’s drug interaction rate closely with inhibition of CYP 2C9, 2D6, and 3A4. Finally, citalopram and escitalopram have the least drug interactions, since they inhibit 2D6 enzymes to a lesser extent.
When considering patient characteristics and safety, SSRIs are safe to use in most patient groups, including those with preexisting cardiac disease, asthma, dementia, and hypertension. The elderly, who are particularly prone to orthostatic hypotension as well as weight loss, may benefit most from using SSRIs since those agents produce weight gain and lack anticholinergic activity.
Tricyclic Antidepressants4,10: TCAs block norepinephrine and serotonin reuptake, but they also have affinity for alpha1, H1, and muscarinic receptors, thus causing anticholinergic adverse effects. There are two subclasses of agents available within this class, the tertiary and secondary amine TCAs (TABLE 2). The secondary amine TCAs (desipramine, maprotiline, nortriptyline) have less affinity for the alpha1, H1, and muscarinic receptors, therefore causing fewer anticholinergic adverse effects than the tertiary amine TCAs (e.g., amitriptyline, amoxapine, clomipramine, doxepin, imipramine).
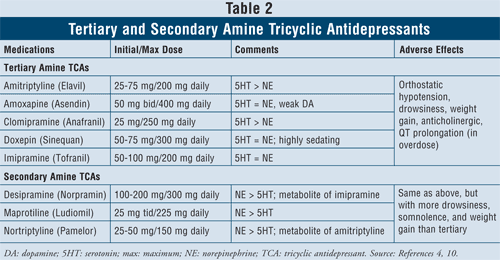
The main adverse effects of this class of agents are orthostatic hypotension, QT prolongation, drowsiness, dry mouth, blurred vision, constipation, and weight gain. In general, tertiary amine TCAs have more serotonin activity versus secondary amine TCAs, which have more norepinephrine activity, thus causing less drowsiness, somnolence, and weight gain.
It is known that TCAs inhibit both CYP 2C19 and 2D6 enzymes.11 They are also metabolized to a lesser extent by CYP 1A2 and 3A4 enzymes.12 For this reason, although drug–drug interactions with these agents might not be the primary concern, it is advised to use caution when combining TCAs with SSRIs, since the drug plasma level of TCAs has the potential to increase by up to fourfold, possibly leading to toxic effects.13,14
When considering patient characteristics and safety, TCAs are contraindicated in some specific cardiac conditions such as patients with a history of arrhythmias, sinus node dysfunction, or conduction defects. Caution is advised with the elderly, as they are more sensitive to cholinergic blockade as well as orthostatic hypotension. Furthermore, individuals also suffering from dementia will be particularly susceptible to the toxic effects of muscarinic blockade on memory and attention span and would generally do best if given antidepressants with the lowest degree of anticholinergic effects. Finally, caution should be exercised when considering starting TCAs in patients with suicidal thoughts due to high lethality risks with overdose. The lethal dose is only eight times the therapeutic dose, so if TCAs are ingested in an overdose, they may block the sinoatrial node in the heart.
Monoamine Oxidase Inhibitors4,10: The MAOIs that are available include isocarboxazid, phenelzine, selegiline, and tranylcypromine (TABLE 3). Aside from selegiline, which is a selective MAO B inhibitor, all other agents inhibit both MAO A and B enzymes responsible for serotonin, norepinephrine, and dopamine metabolism in the brain. Due to severe adverse effects and required diet restrictions, MAOIs are generally reserved for patients who have failed other antidepressants.
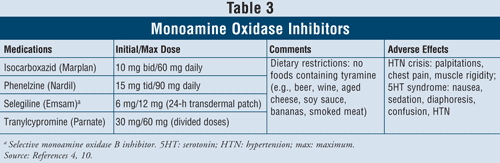
Hypertensive crisis can occur when patients taking MAOIs ingest foods containing tyramine, such as beer, wine, aged cheese, and smoked meat. This reaction presents as an acute onset of severe headache, nausea, neck stiffness, heart palpitations, chest pain, and confusion. MAOIs can also cause serotonin syndrome. As mentioned previously, this syndrome most commonly occurs when MAOIs are taken concomitantly with other serotonergic agents such as SSRIs or if venlafaxine, an SNRI, is administered soon after an MAOI. When patients are switched from an SSRI with a short half-life to an MAOI, it is important that a 2-week washout period be respected between the discontinuation of the SSRI and the start of the MAOI. If fluoxetine is the SSRI, which has a long half-life, the washout period should be 5 weeks.4 Other adverse effects can occur with MAOIs such as orthostatic hypotension, weight gain, sexual dysfunction, and insomnia.
Due to the high rate of drug interactions with these agents, caution should be used when prescribed to patients with asthma using sympathomimetic bronchodilators. In patients with hypertension, MAOIs may induce orthostatic hypotension, especially with concurrent diuretic treatments.
Serotonin-Norepinephrine Reuptake Inhibitors4,10: The SNRIs such as desvenlafaxine, duloxetine, and venlafaxine may also be used as first-line agents (TABLE 4). These medications are safer than TCAs, and their adverse effects are similar to those of SSRIs, including nausea, vomiting, and sexual dysfunction, as well as elevated blood pressure.
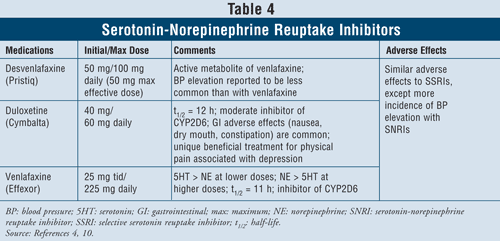
Venlafaxine has been shown to cause an increase in blood pressure in 3% to 13% of cases, while desvenlafaxine was reported to cause an increase in blood pressure in only 1% to 2% of cases.10 Thus, it is recommended to avoid using venlafaxine in patients with uncontrolled hypertension since the agent can exacerbate the condition. Duloxetine has more norepinephrine activity than both of the aforementioned agents, thus being useful with physical symptoms such as muscle aches, headaches, stomach issues, and generalized pain, often occurring with severely depressed patients. Due to its effectiveness in pain symptoms, duloxetine has also been approved for other indications such as fibromyalgia and diabetic peripheral neuropathic pain.15 Finally, all three agents have more serotonin than norepinephrine activity at lower doses and more norepinephrine than serotonin activity at higher doses, thus having dose-dependent adverse effects.
Other Antidepressants4,10: Several other antidepressants are available that differ in their mechanism of action from the classes of medications described previously.
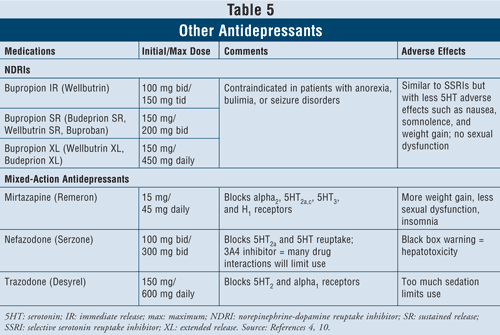
The norepinephrine-dopamine reuptake inhibitors (NDRIs) such as bupropion immediate-release (IR) branded Wellbutrin (also available in long-acting dosage forms such as Wellbutrin XL, Wellbutrin SR, Budeprion SR, Budeprion XL, and Buproban) may be used as first-line agents to treat depression (TABLE 5). Their adverse effects are similar to those of SSRIs, with minimal serotonin effects such as nausea and weight gain and little or no sexual dysfunction. Bupropion has been shown to exert beneficial effects on Parkinson’s disease symptoms in some patients, but it may also induce some psychotic symptoms, perhaps because of its agonistic action on the dopaminergic system.16
Finally, there are three more antidepressants with mixed action available: mirtazapine, nefazodone, and trazodone (TABLE 5). All three agents block different serotonin receptors, thus having distinct effects. Mirtazapine causes more weight gain by increasing appetite. Nefazodone has limited uses because of hepatotoxicity and CYP3A4 enzyme inhibition, which leads to drug interactions. Trazodone blocks serotonin receptors to a great extent, with poor binding to muscarinic receptors. Adverse effects such as sedation, headache, memory impairment, dry mouth, and constipation may occur. Caution is also advised with trazodone use in men due to risk of priapism.
Conclusion
In general, antidepressant medications have been shown to be equally efficacious; therefore, medication choice should be based on adverse effects, drug interactions, safety, and patient preferences. Several algorithms are available to guide the clinician during the patient’s treatment, particularly the recently updated Texas Department of State Health Services algorithm for the treatment of MDD (updated July 2008).17 If patients show partial response, clinicians may choose to increase the dose, change to an alternative agent, or give a combination of antidepressants. On the contrary, if patients do not respond or cannot tolerate the drug, switching to an alternative agent is also appropriate, taking into consideration that therapeutic effects will usually occur between 4 to 6 weeks, even though adverse effects might appear after 1 week of treatment.4,17 In addition, while the adverse effects appear early in treatment, they generally dissipate after 2 to 3 weeks.4,17 Nonetheless, the antidepressant that will most likely ensure a patient’s improvement and safety may be determined, at least partially, by trial and error. Given the difficulty in predicting what medication will be both efficacious for and tolerated by an individual patient, familiarity with a broad spectrum of antidepressants is prudent and useful.
REFERENCES
1. Song F, Freemantle N, Sheldon TA, et al. Selective serotonin reuptake inhibitors: meta-analysis of efficacy and acceptability. BMJ. 1993;306:683-687.
2. Geddes JR, Freemantle N, Mason J, et al. SSRIs versus other antidepressants for depressive disorder. Cochrane Database Syst Rev. 2000;(2):CD001851.
3. Rush AJ, Wisniewski SR, Warden D, et al. Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry. 2008;65:870-881.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
5. Work Group on Major Depressive Disorder. Guideline Watch: Practice Guidelines for the Treatment of Patients with Major Depressive Disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2005.
6. Cain RA. Navigating the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study: practical outcomes and implications for depression treatment in primary care. Prim Care Clin Office Pract. 2007;34:505-519.
7. Depression. World Health Organization. www.who.int/mental_health/
8. Dipiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New York, NY: McGraw-Hill; 2005.
9. Revisions to product labeling. Suicidality and antidepressant drugs. FDA. www.fda.gov/downloads/Drugs/
10. Drug information. Micromedex Healthcare Series. Greenwood Village, CO: Thomson Micromedex. www.micromedex.com. Accessed October 5, 2009.
11. Shin J-G, Park J-Y, Kim M-J, et al. Inhibitory effects of tricyclic antidepressants on human cytochrome P450 enzymes in vitro: mechanism of drug interaction between TCAs and phenytoin. Drug Metab Dispos. 2002;30:1102-1107.
12. Brown CH. Overview of drug–drug interactions with SSRIs. US Pharm. 2008;33(1):HS3-HS19.
13. Spina E, Scordo MG, D’Arrigo C. Metabolic drug interactions with new psychotropic agents. Fundam Clin Pharmacol. 2003;17:517-538.
14. Bergstrom RF, Peyton AL, Lemberger L. Quantification and mechanism of the fluoxetine and tricyclic antidepressant interaction. Clin Pharmacol Ther. 1992;51:239-248.
15. Cymbalta (duloxetine hydrochloride) package insert. Indianapolis, IN: Eli Lilly and Company; February 2009. http://pi.lilly.com/us/
16. Goetz CG, Tanner CM, Klawans HL. Bupropion in Parkinson’s disease. Neurology. 1984;34:1092-1094.
17. Suehs B, Argo TR, Bendele SD, et al. Texas Medication Algorithm Project Procedural Manual. Major Depressive Disorder Algorithms. Texas Department of State Health Services; July 2008. www.dshs.state.tx.us/
mhprograms/pdf/TIMA_MDD_
Manual_080608.pdf. Accessed October 5, 2009.
To comment on this article, contact rdavidson@jobson.com.






