US Pharm. 2008;33(3):59-65.
The Omnibus Budget Reconciliation Act of
1990 (OBRA '90) included mandates for the states to improve understanding of
medications by Medicaid beneficiaries for whom they were prescribed and
dispensed.1 Although the Act was part of legislation passed in
1990, the pharmacy practice requirements did not go into effect until 1993.
While the original statute was aimed at Medicaid recipients, each state had
the option to adopt rules making those provisions applicable to other patients
as well. Some jurisdictions limited the directives to only new prescriptions
whereas others made the obligations applicable to both new and refilled
prescriptions. A patchwork quilt of requirements has also evolved regarding
whether the offer to counsel must be made personally by the pharmacist.
The various mandates of OBRA '90 are
reviewed and the responses of the states after enactment are highlighted. The
OBRA '90 mandates at the state level have played a part in a number of civil
law suits across the nation. Is there a private right of action for patient
injury attributable to a deviation from OBRA '90 mandates? How have courts
sorted through this set of professional obligations for the pharmacist?
Sufficient time has now passed to glean some lessons from these cases. Those
lessons from the past and lessons anticipated in the future will be
emphasized.
The shift in the focus of
pharmacists' education and practice activities from products to patients
gained momentum throughout the 1970s and 1980s, culminating in adoption of a
variety of federal mandates pertaining to pharmacy practice in the 1990 budget
bill enacted by Congress. The Omnibus Budget Reconciliation Act of 1990, known
colloquially as OBRA '90, was enacted toward the end of the Congressional
session to pull together in one piece of legislation all the
appropriations-related authorizations and requirements flowing from the
various Congressional enactments of the 101st Congress.
One of those components was a
set of provisions added to the federal statutory scheme governing federal
participation in the federal-state partnership program officially designated
Grants to States for Medical Assistance Programs, known in everyday parlance
as the Medicaid program. As Title XIX of the Social Security Act, this program
has both federal and state financial components, providing the federal
government with leverage to impose certain mandates on the states in order for
them to get the federal matching funds, which now can exceed 75% of the
program's cost to the state.2
The Statute at the Federal
Level
President George H.W. Bush signed
the bill into law on November 5, 1990, and the effective date of the
requirements related to pharmacy practice was established as January 1, 1993.
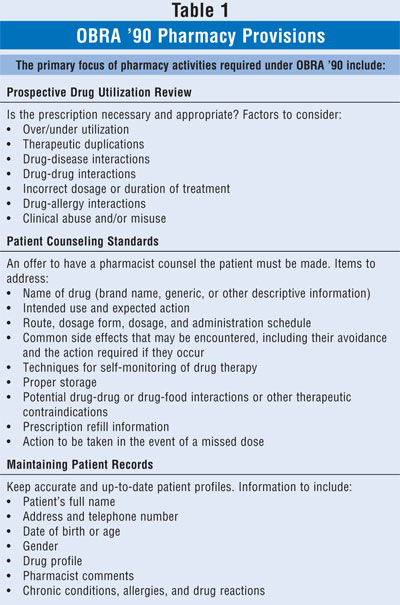
3 The federal statute had several major components: 1) Prospective Drug
Use Review, 2) Retrospective Drug Use Review, 3) Assessment of Drug Use Data,
and 4) Educational Outreach Programs. The first component (Prospective Drug
Use Review) has had the greatest impact on practice of the profession on a
daily basis. TABLE 1 provides a summary of the required pharmacy
activities.
The States Respond with
Statutes or Regulations
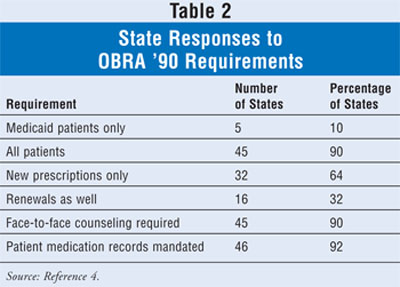
When these mandates
reached the states, officials with the Medicaid program, along with leaders of
the board of pharmacy or a similar administrative agency, began to formulate
their responses. Several decisions needed to be made--the cascading federal
mandate was for Medicaid beneficiaries only, but should this be extended to
all patients? Should the offer-to-counsel requirement be limited to newly
dispensed prescriptions or should it also include renewals? Must the offer to
counsel be extended by a pharmacy staff member or would a sign suffice? Must
the counseling be performed by the pharmacist face to face with the patient?
Should maintenance of patient medication records be mandated? How the states
are currently split on these questions, as reported by state boards of
pharmacy to the National Association of Boards of Pharmacy, is shown in
TABLE 2.4
The Courts Interpret OBRA
'90 in a Variety of Contexts
We can learn a variety of lessons
about how courts view pharmacy and pharmacists from case decisions where the
statutes or regulations were interpreted. But there is an important caveat to
bear in mind. The following cases only address issues considered by appellate
courts in published opinions. Except for cases in federal district courts,
there are no direct means to review decisions by state-level trial courts, as
very few opinions are released or reported in the legal literature.
|
Cases Addressing the Standard of
Care to Be Exercised by Pharmacists
In a case arising
before the January 1, 1993, effective date of the offer-to-counsel mandate,
the Court of Appeals of Georgia ruled that a pharmacist has no duty to warn a
patient of, or to refuse to dispense a medication in light of, a potentially
severe side effect arising from excessive dosage. Summary judgment entered for
the defendant pharmacy was upheld on appeal, but the court noted that this
"would not be controlling precedent for cases involving pharmacists' duties
arising after January 1, 1993."5
In a separate case arising
before the January 1, 1993, effective date of OBRA '90, the Florida Court of
Appeals ruled that a pharmacist who accurately dispensed medication but failed
to alert the patient or prescriber of potentially serious adverse drug
interactions has no duty to warn.6
A pediatric patient was
injured, and the question imposed on the pharmacist was whether the obligation
to offer to counsel the parents as the caregivers and agents of the patient
gives rise to a legal obligation related to the parents for their emotional
well-being, as opposed to the actual patient's well-being. The Supreme Court
of California ruled that no, this would be an "unwarranted enlargement of
potential liability."7
The Missouri Court of Appeals
reversed a summary judgment of the trial court granted in favor of the
defendant pharmacist where the lower court had ruled that the pharmacist's
"only obligation was to fill [sic] the prescriptions accurately." The case
involved a "strong hypnotic drug prescribed at three times the normal dose."
The court said, "Pharmacists are trained to recognize proper dose and
contraindications of prescriptions, and physicians and patients should welcome
their insights to help make the dangers of drug therapy safer." Summary
judgment was overruled and the case returned to the trial court for a decision
on whether the pharmacist met his legal duty under the facts of the case.
8
In another case, a woman
suffering an acute asthma attack entered the pharmacy and requested that the
pharmacist either give her an inhaler, for which she had no prescription on
record at this pharmacy, or call the physician or hospital to obtain a
prescription. The pharmacist would do neither, and the patient was taken by
ambulance to the hospital. The issue here was whether the pharmacist had a
legal duty to act arising from OBRA '90. The court ruled no; the OBRA '90
duties arise after receipt of a "prescription drug order." Absent a
prescription or a preexisting pharmacist-patient relationship, the pharmacist
had no legal duty to act.9
In an officially unpublished
opinion (that was nevertheless reported in a jurisprudence database), a
patient who declined counseling and signed a waiver form was not permitted to
later allege that he should have been counseled anyway. This was a very
interesting case where the patient made all sorts of convoluted arguments as
to why he should be allowed to maintain this claim, but in the end he not only
lost but had to pay the costs of the pharmacy chain's appeal.10
Cases Addressing the Issue
of "Mandatory" Copayments Reducing Reimbursement to Pharmacies
At issue in this
case was the provision in OBRA '90 that "reimbursement limits" may not be
modified by the federal government and that state governments may not "reduce
the limits for covered outpatient drugs" if the state's Medicaid plan was in
compliance with applicable federal regulations when certain emergency
regulations were adopted. The focus here was whether Pennsylvania's plan was
deemed to be "in compliance," meaning that no reduction in reimbursement was
permitted. The court ruled that yes, the state was in compliance, and
therefore was prohibited from reducing payments for covered outpatient
medications.11
The four-year moratorium on
reduction in medication reimbursement limits was also at issue in this case in
a U.S. district court in Florida. After the effective date of OBRA '90, the
Florida legislature passed an appropriations bill that implemented a copayment
feature for the state's Medicaid drug program. Reimbursement rates to
pharmacies were not altered, but the state agency charged with administering
the program automatically reduced payments to providers by the amount of the
copayment. Further, federal program requirements prohibit a participating
provider from denying services to a program participant who cannot pay the
cost-sharing amount. The Florida Pharmacy Association advanced the argument
that this amounted to a reduction in reimbursement, a violation of the OBRA
'90 moratorium. The court did not agree. In its view the pharmacies now have
two sources of reimbursement--state plus patient--rather than one as before,
stating that "whether one of the sources, the recipients, may not be so
forthcoming with reimbursement does not change the fact that the reimbursement
limits have not been changed."12
The copayment implemented for
Medicaid-covered medications in Nebraska presented issues parallel to those in
the Florida case discussed above. Noting that authorization for states to
implement copayments in the Title XIX drug program had been in place since
1982, the court ruled that the defendant state officials "have ordered that in
certain cases a pharmacist may never actually recover the reimbursement limits
notwithstanding the fact that the pharmacist would theoretically be eligible
for maximum reimbursement. The plain words of the federal statute prohibit
this practice." Waxing poetic, the judge in the case continued, "This case
brings to mind Gertrude Stein's familiar words: 'A rose is a rose is a
rose'...A reduction is a reduction, whether by change in a formula or
otherwise."13
Indiana's plan had a 50 cent
copayment for generic medications and a dollar copayment for brand name
products at that time. And the court there quickly grasped the issue. "While
the Moratorium clearly prohibits a decrease in ëreimbursement level,' it does
not explicitly state how the copayment provisions, which predates the
Moratorium, is to be affected." In the end, the request of the Indiana
Pharmacists' Association for an injunction prohibiting such action by the
state government was granted.14
The federal district court in
Indiana noted that "the State is able to reduce its reimbursement liability to
pharmacists not only by the amount of copayments that are collected by
pharmacists, but also by the amount of copayments the pharmacists are unable
to collect because of the patient's inability to pay." It was estimated that
50% of Medicaid recipients could not afford the copayment. The state agency
argued that the federal statute prohibits reducing "payment limits," which it
was not doing; it was reducing the actual amount paid. The court rejected that
interpretation, concluding that "in enacting the moratorium, Congress intended
to protect the actual level of reimbursement flowing to pharmacists."
15
Case Addressing Issues with
Mandatory Rebates from Manufacturers
The Pharmaceutical
Research and Manufacturers of America (PhRMA) challenged the provision in
Michigan's OBRA '90 authorized program whereby states established preferred
drug lists for Medicaid and required prior authorization for coverage of
nonpreferred medications that had generated insufficient rebates for the
state. The state's program was upheld.16
Conclusions
The five cases that
address pharmacy reimbursement are likely last words on that issue based on
OBRA '90 provisions, given that the moratorium on states altering the methods
of calculating fees and benefits has since expired. This is not to say that
there are not still, and probably always will be, reimbursement disputes.
Likewise, rebate issues will linger as well. It has been noted that "as more
courts are asked to address the legal standard of pharmacy practice, they will
turn to OBRA '90 to assist them in defining those standards. OBRA '90 mandates
that pharmacists take on additional responsibilities, and with those expanded
responsibilities come expanded expectations and liability."17
Given that there are only six published opinions that address the standard of
care exercised by pharmacists over the past few years using OBRA '90 language,
it is fair to conclude that this statute has not caused the revolution in
pharmacist counseling that some commentators or observers predicted at the
time of its enactment and implementation. It is commonly observed that the
wheels of justice turn slowly. Given this, there is still hope that OBRA '90
still be considered a planted seed that yields a change in the communications
relationships between pharmacists and patients.
Adapted from a poster presented at the
American Society for Pharmacy Law 32nd Annual Meeting/American Pharmacists
Association 154th Annual Meeting, Atlanta, Georgia, March 16-19, 2007, and
winner of the Research Award from the American Society for Pharmacy Law, 2007.
REFERENCES
1. P.L. 101-508, 104 Stat. 1388, codified at 42 U.S.C. ßß1396r-8g.
2. Ku L, Park E. Federal aid to state Medicaid programs is falling while the economy weakens. Center on Budget and Policy Priorities. October 26, 2001. www.cbpp.org/10-11-01health.htm. Accessed February 26, 2008.
3. Medicaid program. Fed Regist. 1992;57:49397-49401.
4. Survey of Pharmacy Law. Sec. XXIII-Patient Counseling Requirements. Mount Prospect, IL: National Association of Boards of Pharmacy CD-ROM; 2007:75-76.
5. Walker v. Jack Eckerd Corp , 434 S.E.2d 63 (Ga. App. 1993).
6. Johnson v. Walgreen Co., 675 So.2d 1036 (Fla. App. 1996).
7. Huggins v. Longs Drug Stores California, Inc., 862 P.2d 148 (Cal. 1993).
8. Horner v. Spalitto, 1 S.W.3d 519 (Mo. App. 1999).
9. Chiney v. American Drug Stores, Inc., et al., 21 S.W.3d 14 (Mo. App. 2000).
10. Hooper v. Thrifty Payless, Inc ., 2003 Cal. App. Unpub. Lexis 11715 (2002).
11. Pennsylvania Pharmaceutical Association, et al. v. Casey, et al., 800 F.Supp 173 (M.D. Pa. 1992).
12. Florida Pharmacy Association, et al. v. Williams, et al., 871 F.Supp. 1441 (N.D. Fla. 1993).
13. Nebraska Pharmacists Association, Inc, et al. v. Nebraska Department of Social Services, et al., 863 F.Supp 1037 (D. Neb. 1994).
14. Indiana Pharmacists' Association, et al. v. Indiana Family and Social Services Administration, 881 F.Supp. 395 (S.D. Ind. 1994).
15. Pharmaceutical Society of the State of New York, Inc. v. New York State Department of Social Services, et al., 50 F.3d 1168 (2nd Cir 1995).
16. Pharmaceutical Research and Manufacturers of America, et al. v. Thompson, 259 F.Supp.2d 39 (D.D.C. 2003).
17. Fink JL III, Vivian JC,
Bernstein IG, eds. Pharmacy Law Digest. 40th ed. St. Louis, MO: Wolters
Kluwer Health; 2005:98-99.
To comment on this article, contact
editor@uspharmacist.com.






