Parkinson's disease (PD) is
a progressive neurodegenerative disorder affecting more than 1.5 million
Americans older than 50 and most frequently appearing between the ages of 50
and 79.1 More than half of all individuals over age 85 may exhibit
some signs of parkinsonism (see belo
w).2 Age is considered a key risk factor in the development of PD,
and incidence of the disease increases dramatically with age.3
Among all age-groups, incidence of PD is 10 to 20 cases per 100,000 but rises
to about 200 cases per 100,000 among persons
in their 70s and 80s.1
The umbrella term
parkinsonism is often used to encompass PD and related syndromes and
generally refers to the motor picture involving bradykinesia (literally "slow
movement"), rigidity, tremor, and balance and gait problems.4
Secondary parkinsonism, due to other causes or nonidiopathic parkinsonism,
has a different etiology and pathology than does PD. Secondary parkinsonism is
the predominant clinical manifestation of a number of disorders (e.g., brain
tumors near the basal ganglia, atherosclerosis of cerebral vessels, head
trauma, progressive supranuclear palsy).1 Secondary Parkinsonism
may also be caused by toxins and is commonly caused by drugs, especially
antipsychotic agents (table 1; see Drug-Induced Parkinsonism below).

Pathophysiology Summary
The common
pathologic feature in PD and secondary parkinsonism is striatal dopamine
deficiency.1 In patients with PD, cell loss occurs in the
substantia nigra with the formation of Lewy bodies (intracellular neuronal
inclusion bodies). Lewy bodies are not present in secondary parkinsonism;
however, the nigral striatal pathway may be impaired, and nigral cell loss or
loss of striatal cellular elements may occur.1
Clinical Presentation
PD begins subtly
and progresses gradually. In its early stages, signs of the disease may be
difficult to differentiate from those of normal aging.5 Most
patients initially present with a slow, coarse tremor of the hand that occurs
when the muscles are at rest (resting tremor) and causes the fingers to
move across the thumb as if rolling pills (pill rolling) (figure). The
tremor decreases when the hand is moving purposefully, may be worsened by
fatigue or stress, and disappears completely during sleep.6 While
this low-frequency, low-amplitude tremor may progress to the other hand, arms,
jaw, and legs, it may become less obvious with progression of PD. Other main
symptoms of PD include bradykinesia, rigidity (a tightness or increase in
muscle tone while at rest or throughout the entire range of motion of a limb
[i.e., cogwheel rigidity] that may be described as stiffness by the
patient), and postural instability (table 2).5
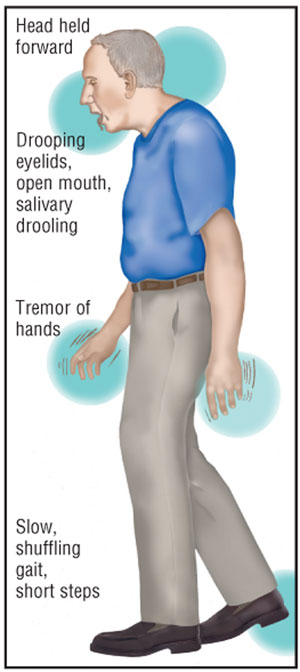
Additional signs may include a
walking pattern that consists of shuffling and short steps without swinging of
the arms. Patients often have difficulty stopping or turning while walking and
may suddenly and unpredictably freeze in place. In contrast, movements may
become unintentionally faster and result in a short-stepped, stumbling run to
avoid falling, while speech may become faster, with words running together in
a mumble.6 The posture of a patient with PD becomes stooped with
the head drooping forward and resting on the chest, which can be dangerous.
When patients lose their balance, their feet and hands cannot move quickly
enough to break a fall.

While the motor symptoms associated
with PD can cause difficulty in performing activities of daily living (ADLs)
such as buttoning clothing and tying shoes, the nonmotor symptoms may have a
greater negative impact on quality of life.3 Nonmotor symptoms
include anxiety, depression, confusion, dementia, urinary dysfunction, and
excessive daytime sleepiness.7-9 Additionally, impairment of the
autonomic nervous system may lead to constipation, orthostatic hypotension,
and excessive sweating.3
PD and Dementia
While there is
significant variation among estimates regarding the frequency of dementia in
PD, approximately 40% to 50% of patients with PD develop dementia, especially
during the late stages of PD and in older individuals.4,5 Even
patients with PD who do not have dementia show slight cognitive deficits.5
Dementia-related problems are usually milder than the dysfunction associated
with Alzheimer's disease and are considered part of the path ology of PD.
4 Characteristics seen in patients with both PD and dementia include
visuospatial def icits, difficulty planning, impaired attention, slowed speed
of processing information, and mildly impaired memory recall; memory
impairment is usually attributed to a retrieval deficit, since recognition is
generally unaffected.5 Some language skills (e.g., vocabulary) are
not affected, while others (e.g., verbal fluency, mechanical aspects of
speech) are impaired.5 Confusion often becomes problematic and is
usually worsened by antiparkinson medications.
Drug- and Toxin-Induced
Parkinsonism
The wide use of
antipsychotic agents in elderly patients for the management of behavioral
problems has resulted in the recognition of antidopaminergic-related adverse
effects, including extrapyramidal signs and symptoms.10 These
drug-related symptoms may be misdiagnosed as a new medical condition (i.e.,
PD) and the patient may be started on antiparkinsonian therapy, thus becoming
vulnerable to additional adverse effects (e.g., delirium, hypotension).10
Discontinuing or reducing the antipsychotic agent is a more desirable
approach. If a neuroleptic is deemed necessary, an agent with a more favorable
adverse effect profile at the lowest possible dose is recommended.10
This also applies to the newer, atypical antipsychotic agents that have
adverse effects at higher doses.
Parkinsonism can be caused by
other agents such as metoclopra mide, methyldopa, and reserpine (all
reversible and dose-dependent), in addition to the meperidine analog, MPTP,
that can cause irreversible parkinsonism in intravenous drug abusers.1
The toxin carbon monoxide can induce irreversible parkinsonism, and manganese
may induce dystonias and cognitive changes with occupationally related chronic
intoxication.1
Medications to Avoid in the
Elderly PD Patient
Symptoms of PD will
worsen with medications that block dopamine receptors, such as antipsychotic
agents (especially the typical antipsychotic agents including haloperidol and
chlorpromazine), the antiemetic prochlorperazine, and the gastrointestinal
prokinetic agent metoclopramide. Large doses of vitamin B6
(pyridoxine) should be avoided to prevent the peripheral conversion of
levodopa (L-dopa) to dopamine, which interferes with the efficacy of L-dopa
therapy. Patients receiving selegiline therapy, associated with an amphetamine
metabolite, should not take meperidine, due to interaction and such risks as
overstimulation of the central nervous system (CNS), seizure, hyperpyrexia,
hypertension, and hypotension. Ephedrine-like prescription and
over-the-counter products should also generally be avoided in these patients.
Anticholinergics should always be used cautiously and are best avoided in the
elderly.
Pharmacotherapy
PD and secondary
parkinsonism that is not drug-induced is incurable. Drugs that cause or
exacerbate parkinsonism should be discontinued. Patients may not require
treatment in the early stages of PD if symptoms do not cause functional
impairment.9,11 The patient should clearly be involved in deciding
when to initiate therapy. The patient's occupation and ADLs, as well as the
risks and benefits of therapy, should be considered. As PD progresses, therapy
becomes more complex, requiring dose adjustments, poly pharmacy, and the use
of rescue treatments.12 Antiparkinson agents (table 1) tend to
cause confusion and toxic psychosis in elderly patients. For this reason, it
is generally recommended that the therapeutic regimen be kept as simple as
possible, since the risk of adverse effects is lower when one or two agents
are given at higher doses as compared to a multiple-drug regimen using lower
doses.1
Amantadine may be used as
monotherapy for up to 12 months before the initiation of L-dopa to target mild
symptoms including tremor and to reduce L-dopa–induced dyskinesias in
later disease.3,9 While early dopamine agonist monotherapy has been
shown to reduce the subsequent risk of dyskinesias and other motor
complications in comparison to L-dopa, it has the potential to cause
orthostatic hypotension and neuropsychiatric adverse effects (e.g., confusion,
hallucinations).3,9 As a result, these agents should be avoided in
patients with confusion, memory or cognitive impairment, and in patients at
risk of hypotension.3,13
While anticholinergics improve
motor symptoms in some patients with PD (especially younger persons with
resting tremor as a predominant symptom), these drugs often produce
constipation, sedation, confusion, urinary retention, dry mouth, and blurred
vision in the elderly.1,3 Furthermore, they are contraindicated in
individuals with glaucoma, benign prostatic hypertrophy, and dementia.1
Dopamine replacement is
accomplished with L-dopa, which should be added to the drug regimen when PD
symptoms can no longer be managed optimally with other agents. Since L-dopa is
converted to dopamine in the CNS and the peri phery, peripheral conversion
and systemic effects can be reduced by combining L-dopa with carbi dopa (a
peripheral decarboxylation inhibitor), which does not cross the blood–brain
barrier.1
The addition of a
catechol-O-methyltransferase (COMT) inhib itor decreases the end-of-dose
failure or "wearing off" of L-dopa therapy that causes motor complications. By
reducing the peripheral metabolism of L-dopa, a COMT inhibitor allows for the
reduction of L-dopa doses.3 Compared with standard L-dopa therapy,
some researchers recommend the initiation of a COMT inhibitor at the onset of
L-dopa therapy to reduce the risk of developing motor complications.
In the later stages of PD,
dopamine agonists may be added to L-dopa therapy in the appropriate patients,
providing greater efficacy and reduced motor complications (as compared to
L-dopa monotherapy) due to the ability to lower the L-dopa dose. Patients who
have a deteriorating response to L-dopa, experience fluctuations in response
to L-dopa, or have a limited clinical response to L-dopa secondary to an
inability to tolerate higher doses are appropriate candidates.14 A
decrease in the frequency of "off" periods and a L-dopa–sparing
effect can occur with dopamine agonists. Apomorphine, a recently FDA-appro
ved, short-acting, dopamine agonist, is delivered by subcutaneous injection as
a rescue dose to manage the sudden and refractory motor fluctuations of
L-dopa–induced "off" periods in patients with PD.3
Its administration is routinely preceded by the antiemetic trimethobenzamide
hydrochloride to prevent adverse effects such as nausea and vomiting.3,9
If patients continue to
experience unpredictable "on" and "off" periods, a monoamine oxidase type B
(MAO-B) inhibitor or amantadine may be added to the regimen; apomorphine may
also be utilized as rescue therapy. Studies on the neuroprotective effects of
MAO-B inhibitors have been inconclusive.3,14
When confusion and
disorientation occurs, discontinuing or lowering the doses of antiparkinsonian
drugs is recommended as follows: anticholinergic agents discontinued first,
followed by selegiline, dopamine agonists, and L-dopa. The pharmacist plays a
critical role in advocating treatment regimens that address administration
needs while keeping patient care a priority.15
Primary Symptoms Related to
Treatment
Dyskinesias:
A complication of L-dopa therapy is dyskinesias--abnormal, choreiform, and
involuntary movements usually involving the neck, trunk, and upper
extremities. Dyskinesias are usually associated with peak antiparkinsonian
benefit, although they can also develop during the rise and fall of L-dopa
effects.14 Dyskinesias can be thought of as too much movement
secondary to the extension of the pharmacologic effect or too much striatal
dopamine receptor stimulation.14 Dyskinesias are more likely to
occur with L-dopa therapy (D1 and D2 agonism) than with
dopamine agonist therapy (primarily D2 agonism), suggesting D1
receptor involvement in producing dyskinesia.14 While most
patients do not mind mild choreiform movements as a trade-off for good
mobility, troublesome dyskinesias should be addressed with strategies aimed at
reducing the amount of L-dopa at each dose, as mentioned above, including
fractioning L-dopa into smaller but more frequent dosages.4,12
End-of-dose failure and
the "wearing off" phenomenon:
In elderly persons, 30% of hospital admissions may be linked to drug-related
problems, and falls leading to hospitalizations are especially likely in
patients with PD, particularly those experiencing "wearing off," a loss of
benefit from a dose of L-dopa that typically occurs after a few hours.16
End-of-dose "wearing off" or deterioration has been related to
increasing loss of neuronal storage capability for dopamine.14
Motor fluctuations may become more severe and dyskinesias occurring during
peak dose effect may occur. Modified treatment strategies (mentioned above)
should be considered at this point to improve symptoms and allow for reduced
doses of L-dopa.15
Surgery
Information on
surgical procedures to relieve symptoms of PD may be obtained by contacting
the American Parkinson Disease Association Inc. at www.apdaparkinson.org, via
email at apda@apdaparkinson.org, or by calling (800) 223-2732.
Diet
Since protein
ingestion interferes with L-dopa absorption, rearranging the timing of
protein-containing meals so that they are consumed in the evening may prevent
interference with L-dopa therapy in patients with advanced PD; agents other
than L-dopa are not affected by protein ingestion.4
Constipation secondary to
L-dopa, physical inactivity, and parkinsonism itself may be addressed through
dietary intake of high-fiber foods, fruits, prunes juice, and other liquids.
Treatment with senna concentrate, one to six 187-mg tablets/day, is effective
on a routine basis versus allowing constipation to become severe.1
New findings indicate that
several dietary risk factors for age-related diseases, such as cardiovascular
disease, cancer, and diabetes, are also risk factors for PD, Alzheimer's
disease, and stroke.17 Dietary manipulations through dietary
restriction and supplementation with folic acid and antioxidants (e.g.,
N-acetylcysteine, acetyl-L-carnitine, alpha-lipoic acid, glutathione,
Ginkgo biloba) to promote successful brain aging are being studied.
17,18
Alternative Interventions
Physical therapy
reduces disabilities from associated symptoms. Acupuncture has been used to
treat dementia under the premise of enhancement of qi (i.e., vital
energy) and blood circulation; literature suggests acupuncture may exert
beneficial effects in patients with vascular dementia and PD.18
Relaxation techniques (e.g., deep-breathing) and music therapy can be useful
to alleviate emotional and psychological concerns of elderly patients, which
may in turn help their memory retention skills.18
On the Horizon
Rasagiline, a new
MAO-B inhibitor currently awaiting FDA approval, has demonstrated monotherapy
efficacy in early PD and adjunctive efficacy to L-dopa therapy in later PD.
19 This agent may interact with fluoxetine, meperidine, and tricyclic
antidepressants. It is metabolized by cytochromes P-3A4 and P-2D6.20
The Committee to Identify
Neuroprotective Agents for Parkinson's has identified a number of compounds as
candidates for further study. Of these, minocycline, creatine, CoQ10, and
GPI1485 have been selected for testing in the Neuroprotective Clinical Trial.
21 Researchers are examining naturally occurring enzymes that appear to
deactivate free radicals, which some scientists think may be linked to the
nerve damage in PD and other neurological disorders.22
Conclusion
To maintain optimal
mobility and enhance the quality of life of patients with PD, it is essential
that the therapeutic approach be tailored to the individual. As PD progresses,
therapy becomes more complex, often requiring dose adjustments, polypharmacy,
and the use of rescue treatments. Pharmacists with knowledge of PD and its
treatment strategies have the opportunity to serve the patient and become an
integral part of the interdisciplinary treatment team.
REFERENCES
1. Beers MH, Berkow
R, eds. The Merck Manual of Geriatrics. 3rd ed. Whitehouse Station, NJ:
Merck & Co; 2000:432-441.
2. Aminoff MJ.
Parkinson's disease and other extrapyramidal disorders. In: Fauci AS,
Braunwald E, Isselbacher KJ, et al. Harrison's Principles of Internal
Medicine. Vol. 2, 14th ed. New York, NY: McGraw-Hill; 1998.
3. Isaacson SH.
Parkinson's disease: an overview of current treatment options.
Consult Pharm. 2005;supp B:S6-S14.
4. Parkinson's Disease
Handbook: A guide for patients and their families. American Parkinson Disease
Association, Inc. 2005.
5. Craft S, Cholerton
B, Reger M. Aging and cognition: what is normal? In: Hazzard WR, Blass JP,
Halter JB, et al. Principles of Geriatric Medicine and Gerontology. 5th
ed. New York: McGraw-Hill, Inc; 2003:1355-1372.
6. Beers MH, Jones TV,
Berkwits M, et al, eds. The Merck Manual of Health & Aging.
Whitehouse Station, NJ: Merck Research Laboratories; 2004:358-369.
7. Marjama-Lyons JM,
Koller WC. Parkinson's disease: update in diagnosis and symptom management.
Geriatrics. 2001;56:24-25.
8. Lang AE, Lozano AM.
Parkinson's disease: first of two parts. N Engl J Med.
1998;339:1044-1053.
9. Koller WC, Silver
DE, Lieberman A. An algorithm for the management of Parkinson's disease.
Neurology. 1994;44(12 suppl 10):S1-S52.
10. Rochon PA, Gurwitz
JH. Medication use. In: Hazzard WR, Blass JP, Halter JB, et al. Principles
of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill, Inc;
2003:219-230.
11. Marsden CD.
Problems with long-term levodopa therapy for Parkinson's disease.
Clin Neuropharmacol. 1994;17:S32-S44.
12. Chen JJ. Management
of wearing off in Parkinson's disease. Consult Pharm. 2005;supp
B:S15-S21.
13. Rascol O, Brooks
DJ, Korczyn AD, et al. A five-year study of the incidence of dyskinesia in
patients with early Parkinson's disease who were treated with ropinirole or
levodopa. N Engl J Med. 2000;342:1484-1491.
14. Nelson MV, Berchou
RC, LeWitt PA. Parkinson's disease. In: DiPiro JT, Talbert RL, Yee GC, et al,
eds. Pharmacotherapy: A Pathophysiologic Approach. 5th ed. New York:
McGraw-Hill; 2002:1089-1102.
15. Chen JJ. The role
of the consultant pharmacist in Parkinson's disease. Consult Pharm
. 2005;supp B:S22-S25.
16. Woodford H, Walker
R. Emergency hospital admissions in idiopathic Parkinson's disease.
Mov Disord. 2005;20:1104-1108.
17. Mattson MP.
Cellular and neurochemical aspects of the aging brain. In: Hazzard WR, Blass
JP, Halter JB, et al. Principles of Geriatric Medicine and Gerontology.
5th ed. New York: McGraw-Hill, Inc; 2003:1341-1354.
18. Wertkin AD, Cizza
G, Blackman MR. Complementary and alternative medicine in aging. In: Hazzard
WR, Blass JP, Halter JB, et al. Principles of Geriatric Medicine and
Gerontology. 5th ed. New York: McGraw-Hill, Inc; 2003:231-242.
19. Rascol O, Brooks
DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with
Parkinson's disease and motor fluctuations (LARGO, Lasting effect in Adjunct
therapy with Rasagiline Given Once daily, study): a randomised, double-blind,
parallel-group trial. Lancet. 2005;365:947-954.
20. Rasagiline
Information Summary: National Institute of Neurological Disorders and Stroke.
Available at:
ninds.nih.gov/funding/research/parkinsonsweb/drug_summaries/rasagiline.htm.
Accessed January 23, 2006.
21. Neuroprotective
Agents for Clinical Trials: National Institute of Neurological Disorders and
Stroke. Available at: ninds.nih.gov/funding/research/
parkinsonsweb/drug_summaries. Accessed January 23, 2006.
22. Henkel J.
Parkinson's Disease: New Treatments Slow Onslaught of Symptoms. U.S. Food and
Drug Administration. Available at: fda.gov/fdac/features/1998/498_pd.html.
Accessed January 9, 2006.
23. Drug Facts and
Comparisons 2005. 59th ed. Facts &Comparisons; 2004.
24. Semla TP, Beizer
JL, Higbee MD. Geriatric Dosage Handbook. 10th ed. Cleveland, Ohio:
Lexi-Comp, Inc; 2005:1404.
To comment on this article,
contact
editor@uspharmacist.com.






