 |
|
|
 |
Please click here to view Full Prescribing Information:
VICODIN 5 mg (hydrocodone)/500 mg (acetaminophen)
VICODIN ES 7.5 mg (hydrocodone)/750 mg (acetaminophen)
VICODIN HP 10 mg (hydrocodone)/660 mg (acetaminophen)
VICODIN, VICODIN ES, VICODIN HP 5, 7.5, 10 mg (hydrocodone)/300 mg (acetaminophen)
Please click here to view Important Safety Information.
|
| |
|
| |
|
| |
 |
REFORMULATION AND
DISCONTINUATION ANNOUNCEMENT |
 |
| |
|
| Introduction of newly reformulated VICODIN® (hydrocodone bitartrate and acetaminophen tablets, USP) and discontinuation of current formulations of VICODIN. |
|
| |
|
|
|
|
| |
|
|
| |
|
|
| |
Dear Healthcare Provider: |
|
| |
On January 13, 2011, the U.S. Food and Drug Administration (FDA) asked drug manufacturers to limit the strength of acetaminophen in prescription drug products, including combination acetaminophen and opioid products, to no more than 325 mg per dosage unit. The FDA has stated that limiting the amount of acetaminophen per dosage unit in prescription products may reduce the risk of severe liver injury from acetaminophen overdosing.1 (Please visit the FDA website for more information on acetaminophen dosing: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm.) |
|
| |
|
|
| |
Introduction of New Formulations |
|
| |
The FDA requested this change be effected by January 2014. Abbott is complying with this directive ahead of the FDA’s requested date and will introduce new formulations of VICODIN® (hydrocodone bitartrate and acetaminophen tablets, USP) with reduced acetaminophen content in the third quarter of 2012. |
|
| |
|
|
| |
In the third quarter of 2012, VICODIN will be available in the following new formulations:
• VICODIN® (hydrocodone bitartrate and acetaminophen tablets, USP) 5 mg/300 mg
• VICODIN ES® (hydrocodone bitartrate and acetaminophen tablets, USP) 7.5 mg/300 mg
• VICODIN HP® (hydrocodone bitartrate and acetaminophen tablets, USP) 10 mg/300 mg
|
|
| |
|
|
| |
Discontinuation of Current Formulations |
|
| |
In order to facilitate this transition, beginning immediately, Abbott is discontinuing the manufacturing and distribution of the current formulations of VICODIN. This is intended to provide sufficient time for product returns, which may minimize confusion and the potential for dispensing errors when new formulations are introduced. Please see below for additional detail on how to return any unexpired VICODIN you may have
in stock.
In addition, Abbott is proactively communicating with prescribers about the changes to VICODIN formulations. |
|
| |
|
|
| |
How to Return Product |
|
| |
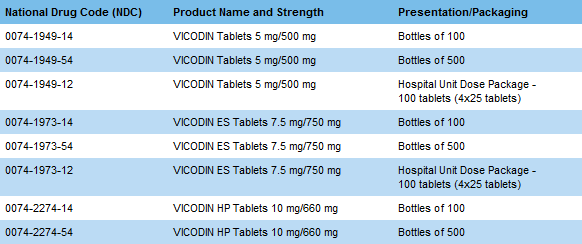
Return of VICODIN products is voluntary. If you choose to return the VICODIN products listed below, Abbott will issue credit to you through your wholesaler for full or partial bottles of product in original Abbott containers that have not expired. No special authorizations from Abbott are required. |
|
| |
|
|
| |
Returns should be sent to:
GENCO
6101 North 64th Street
Milwaukee, WI 53218 |
|
| |
|
|
| |
 |
|
| |
|
|
| |
If you have any further questions regarding returns of this product, you may call Abbott Customer Service at 1-800-255-5162. |
|
| |
|
|
| |
Considerations for Healthcare Providers |
|
| |
In the interim period between the discontinuation of the current VICODIN formulations and the introduction of the new VICODIN formulations, please work with your patients to ensure their pain is managed with the appropriate medication.
The reformulation and discontinuation only affect the VICODIN family of products (distributed by
Abbott Laboratories). |
|
| |
|
|
| |
Reporting Adverse Events |
|
| |
You can assist us with monitoring the safety of VICODIN by reporting adverse events to Abbott at
1-800-633-9110. Alternatively, this information may be reported to the FDA's MedWatch reporting system by phone (1-800-FDA-1088), fax (1-800-FDA-0178), www.fda.gov/medwatch, or mailed to: MedWatch, HF-2, 5600 Fishers Lane, Rockville MD, 20852-9787. |
|
| |
|
|
| |
For More Information |
|
| |
Should you have any questions or require further information regarding VICODIN, the Abbott Medical Information Department may be reached at 1-800-633-9110 or via e-mail at medinfo@abbott.com. |
|
 |
 |
 |
 |
Patient Communication2-5 |
|
| |
• Patients should not take more than 4000 milligrams of acetaminophen per day.
• Patients should be instructed to call the physician if they have taken more than the recommended dose. |
|
| |
|
|
|
|
| |
Indication2-5 |
|
| |
VICODIN® 5 mg/300 mg,
VICODIN ES® 7.5 mg/300 mg,
VICODIN HP® 10 mg/300 mg,
VICODIN® 5 mg/500 mg,
VICODIN ES® 7.5 mg/750 mg,
and VICODIN HP® 10 mg/660 mg (hydrocodone bitartrate and acetaminophen tablets, USP) tablets are indicated for the relief of moderate to moderately severe pain. |
|
| |
|
|
| |
Important Safety Information2-5 |
|
| |
|
|
| |
BOXED WARNING |
|
| |
|
|
| |
HEPATOTOXICITY: ACETAMINOPHEN HAS BEEN ASSOCIATED WITH CASES OF ACUTE LIVER FAILURE, AT TIMES RESULTING IN LIVER TRANSPLANT AND DEATH. MOST OF THE CASES OF LIVER INJURY ARE ASSOCIATED WITH THE USE OF ACETAMINOPHEN AT DOSES THAT EXCEED 4000 MILLIGRAMS PER DAY, AND OFTEN INVOLVE MORE THAN ONE ACETAMINOPHEN-CONTAINING PRODUCT. |
|
| |
|
|
| |
CONTRAINDICATIONS |
|
| |
VICODIN, VICODIN ES, and VICODIN HP tablets are contraindicated in patients previously exhibiting hypersensitivity to hydrocodone or acetaminophen, and also in patients known to be hypersensitive to other opioids, as they may exhibit cross-sensitivity to hydrocodone. |
|
| |
|
|
| |
WARNINGS |
|
| |
Controlled Substance: VICODIN, VICODIN ES, and VICODIN HP contain hydrocodone, which is an opioid agonist and a Schedule III controlled substance with an abuse liability. |
|
| |
|
|
| |
Abuse and Dependence: VICODIN, VICODIN ES, and VICODIN HP can be abused in a manner similar to other opioid agonists, legal or illicit. Psychological dependence, physical dependence, and tolerance may develop upon repeated administration of narcotics; therefore, these products should be prescribed and administered with caution. |
|
| |
|
|
| |
Hypersensitivity/Anaphylaxis: There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. |
|
| |
|
|
| |
Respiratory Depression: At high doses or in sensitive patients, hydrocodone may produce dose-related respiratory depression. |
|
| |
|
|
| |
Head Injury and Increased Intracranial Pressure: The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury or other intracranial pressure. |
|
| |
|
|
| |
Acute Abdominal Conditions: The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions. |
|
| |
|
|
| |
PRECAUTIONS |
|
| |
As with any narcotic, special caution should be used when prescribing hydrocodone to elderly or debilitated patients and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy, or urethral stricture. Caution should also be exercised with patients who are likely to take other acetaminophen-containing medications, antihistamines, antipsychotics, antianxiety agents, other
narcotic analgesics, or other central nervous system (CNS) depressants (including alcohol) concomitantly. When combined therapy is contemplated, the dose of one or both agents should be reduced. Hydrocodone, like all narcotics, may impair mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery.
The use of monoamine oxidase (MAO) inhibitors or tricyclic antidepressants with hydrocodone preparations may increase the effect of either the antidepressant or hydrocodone.
VICODIN, VICODIN ES, and VICODIN HP tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. Administration to the mother during labor or shortly before delivery may result in some degree of respiratory depression in the newborn. |
|
| |
|
|
| |
ADVERSE REACTIONS |
|
| |
The most frequently reported adverse reactions include lightheadedness, dizziness, sedation, nausea, and vomiting. Prolonged administration may produce constipation. |
|
 |
 |
 |
| |
|
|
|
|
| |
DOSAGE AND ADMINISTRATION |
|
| |
• VICODIN 5 mg/300 mg: The usual adult dosage is one or two tablets every four to six hours as needed
for pain. The total daily dosage should not exceed 8 tablets.
• VICODIN ES 7.5 mg/300 mg: The usual adult dosage is one tablet every four to six hours as needed for
pain. The total daily dosage should not exceed 6 tablets.
• VICODIN HP 10 mg/300 mg: The usual adult dosage is one tablet every four to six hours as needed for
pain. The total daily dosage should not exceed 6 tablets.
|
|
 |
 |
 |
| |
|
|
|
|
| |
DOSAGE AND ADMINISTRATION |
|
| |
• VICODIN 5 mg/500 mg: The usual adult dosage is one or two tablets every four to six hours as needed
for pain. The total daily dosage should not exceed 8 tablets.
• VICODIN ES 7.5 mg/750 mg: The usual adult dosage is one tablet every four to six hours as needed for
pain. The total daily dosage should not exceed 5 tablets.
•
VICODIN HP 10 mg/660 mg: The usual adult dosage is one tablet every four to six hours as needed for
pain. The total daily dosage should not exceed 6 tablets. |
|
| |
|
|
| |
Sincerely, |
|
| |
 |
|
| |
Robert Hoff, MD
Head
Global Medical Communications |
|
| |
|
|
| |
Please click here to view Full Prescribing Information:
VICODIN 5 mg (hydrocodone)/500 mg (acetaminophen)
VICODIN ES 7.5 mg (hydrocodone)/750 mg (acetaminophen)
VICODIN HP 10 mg (hydrocodone)/660 mg (acetaminophen)
VICODIN, VICODIN ES, VICODIN HP 5, 7.5, 10 mg (hydrocodone)/300 mg (acetaminophen) |
|
| |
|
|
| |
Please see Important Safety Information, including BOXED WARNING on hepatotoxicity. |
|
| |
|
|
| |
For more information, visit www.Vicodin.com. |
|
| |
|
|
| |
References: |
|
| |
| 1. |
United States Department of Health and Human Services. Information by Drug Class: Acetaminophen. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Accessed October 28, 2011. |
| 2. |
VICODIN 5 mg (hydrocodone)/500 mg (acetaminophen) [package insert]. North Chicago, IL: Abbott Laboratories. |
| 3. |
VICODIN ES 7.5 mg (hydrocodone)/750 mg (acetaminophen) [package insert]. North Chicago, IL: Abbott Laboratories. |
| 4. |
VICODIN HP 10 mg (hydrocodone)/660 mg (acetaminophen) [package insert]. North Chicago, IL: Abbott Laboratories. |
| 5. |
VICODIN, VICODIN ES, VICODIN HP 5, 7.5, 10 mg (hydrocodone)/300 mg (acetaminophen) [package insert]. North Chicago, IL: Abbott Laboratories. |
|
|
| |
|
|
| |
We respect your privacy. For information, please view our privacy policy. PLEASE DO NOT REPLY TO THIS MESSAGE. If you have questions or comments, contact Abbott Customer Service at https://www.abbott.com/abtcontactus/contact.do?BUID=114000&CUID=825004. |
|
| |
|
|
| |
Mail written correspondence to:
Abbott Consumer Relations
100 Abbott Park Road
Abbott Park, IL 60064-3500 |
|
| |
|
|
| |
©2012 Abbott Laboratories Abbott Park, IL 60064 084-825004 April 2012 |
|
| |
|
|
| |
|
|
 |
 |
 |
|
|
|

