US Pharm. 2018;43(7):15-21.
Special thanks to the Global Initiative for Asthma (GINA) for permission to use their 2018 report findings cited in this article.
ABSTRACT: Asthma is a lifelong respiratory disease that calls for proper pharmacotherapy, patient counseling, and an action plan designed to reduce the frequency and severity of asthmatic symptoms. The overall goal of pharmacotherapy is to provide relief for acute episodes while taking preventive measures to reduce the risk of future incidents. Although death from asthma is uncommon, it is estimated that nearly one-half of all asthma patients do not have proper control over their condition. Patient education remains the most crucial element for achieving the desired goals of treating and managing asthma.
Asthma is a lifelong respiratory disease that requires proper pharmacotherapy, patient counseling, and an action plan designed to reduce the frequency and severity of asthma symptoms. Although death from asthma is uncommon, it is estimated that nearly one-half of all asthma patients do not have proper control over their condition.
PATHOLOGY
The pathology of asthma derives from a combination of genetics and exaggerated allergic responses to a variety of possible irritants such as aeropollutants, cold air, exercise, and emotional stress.1 The introduction of an allergen begins a cascade in which mast cells and macrophages release inflammatory mediators such as histamine, leukotrienes, and cytokines. These agents cause mucus production and bronchiole constriction, resulting in labored breathing and/or wheezing. Over time, inflammatory cells can affect growth factors related to bronchiole development, leading to remodeling of the airways and subsequent chronic asthma.2
DIAGNOSIS
Evidence of excessive variability in expiratory lung function is necessary to confirm a diagnosis of asthma.3 Testing includes the analysis of forced expiratory volume over 1 second (FEV1) and forced vital capacity (FVC), a measure of the total amount of air exhaled. In the nonasthmatic population, the FEV1/FVC ratio is normally greater than 0.75 to 0.8 in adults and 0.9 in children; lower values less indicate airway obstruction.
An asthma diagnosis can be confirmed by a positive bronchodilator test. This test may be performed while symptoms are present or early in the morning. For adults, 200 to 400 mcg of albuterol or equivalent should be administered and FEV1 retested 10 to 15 minutes afterward. If FEV1 has increased more than 12% and 200 mL from baseline, the diagnosis of asthma is confirmed. For children, diagnosis is confirmed if FEV1 is greater than 12% of the predicted value after albuterol treatment.3
Peak expiratory flow is another test that may be used, but it is less reliable because of significant differences in interdevice measurements.3
PHARMACOTHERAPY
Pharmacotherapy for asthma may be separated into three categories: controller medications, reliever medications, and add-on therapies (for severe or refractory asthma). The overall goal is to provide relief from acute episodes while taking preventive measures to reduce the risk of future incidents. The Global Initiative for Asthma (GINA) collaborates with the National Heart, Blood, and Lung Institute and the World Health Organization to develop guidelines to treat and manage asthma with a stepwise approach.3
Adults, Adolescents, and Children Aged 6 to 11 Years
FIGURE 1 outlines proper pharmacotherapy corresponding to asthma severity in patients aged 6 years through adults.2 Step 1 from the GINA guidelines warrants the consideration of low-dose inhaled corticosteroids (ICS; agents listed in TABLE 1).
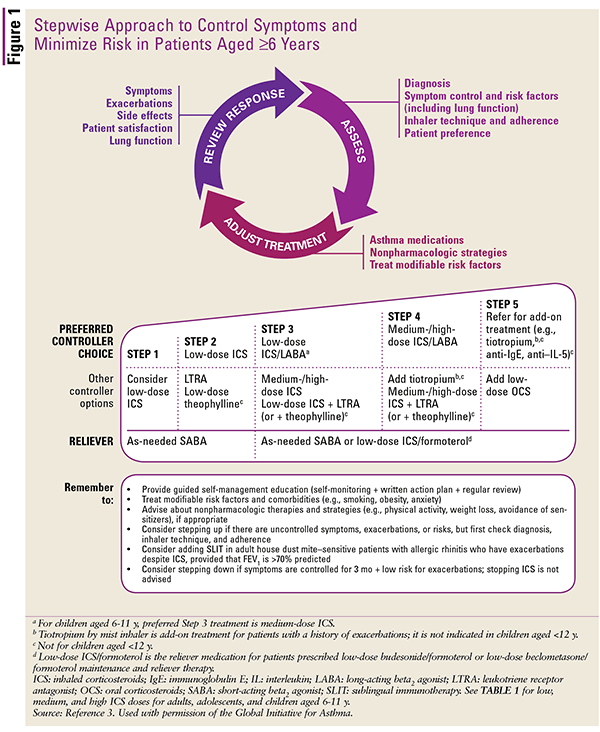
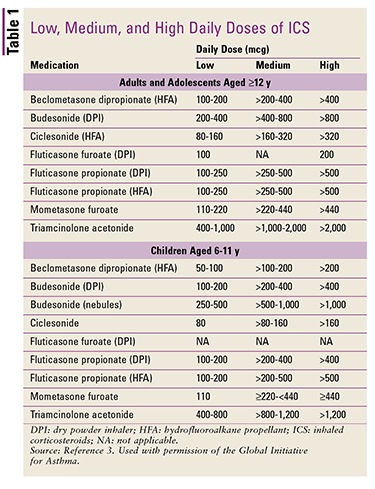
Patients older than 6 years should be assessed via spirometry (FEV1/FVC) every 1 to 3 months. Once an effective treatment regimen has been determined, stepping-down of therapy should be considered until the minimum effective treatment has been obtained. Regardless of asthma severity, all patients should be prescribed a short-acting beta2 agonist (SABA; agents listed in TABLE 2) as a reliever agent during acute asthma exacerbations, and it should be carried at all times.3
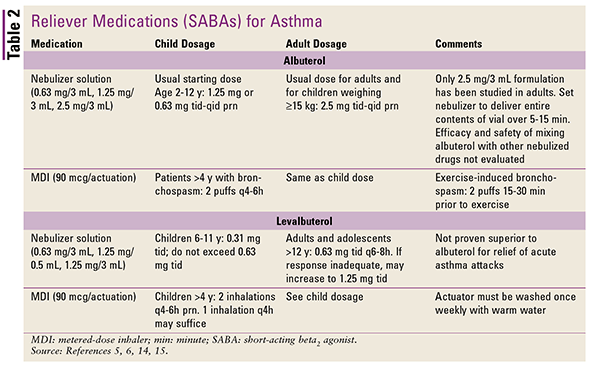
SABAs exhibit their therapeutic effects by lowering intracellular calcium concentrations, leading to relaxation of the smooth muscles in the airways.5 Albuterol products are racemic, 50:50 mixtures of the S and R isomers. The pure R isomer, which is sold as levalbuterol, has not proven superior to racemic albuterol.6 Caution should be used in patients with cardiac arrhythmia, as SABAs have been shown to cause tachycardia.
Epinephrine may be employed as an adjunct to treat severe exacerbations, but it is not recommended for routine use.3 SC terbutaline may also be used for such exacerbations, but with close monitoring.
Ipratropium is often employed as a reliever medication, either alone or in combination with albuterol. In adults, the albuterol combination has been shown to provide greater relief from acute exacerbations than albuterol alone. It may also be considered during emergency situations in which albuterol is not effective and when side effects of albuterol, such as tachycardia, must be avoided.3
In Step 2 of the GINA guidelines, low-dose ICS are added, with the consideration of adding a leukotriene-receptor antagonist (LTRA) or low-dose theophylline.3 However, theophylline is rarely used in current practice and is involved in several drug interactions, resulting in a significant increase of potentially harmful theophylline levels; it should be used with extreme caution in patients with peptic ulcer disease, seizure disorders, and cardiac arrhythmias.7
Montelukast exhibits its therapeutic effect by preventing leukotriene D4 from binding to the cysteinyl leukotriene receptor 1, thereby decreasing bronchiolar edema and smooth-muscle contraction. It is available as tablets, chewable tablets, and granule packets.8 Montelukast requires once-daily evening dosing. Zafirlukast, another LTRA approved for asthma, is available only in tablets and requires twice-daily dosing; it also has been associated with severe hepatotoxicity.9
Step 3 involves the addition of a long-acting beta2 agonist (LABA) with an ICS as a combination product (TABLE 3).3 Another controller choice is use of a medium- to high-dose ICS, or the option from Step 2 but with a higher dose of an LTRA. The reliever regimen calls for an as-needed SABA or low-dose ICS/formoterol.
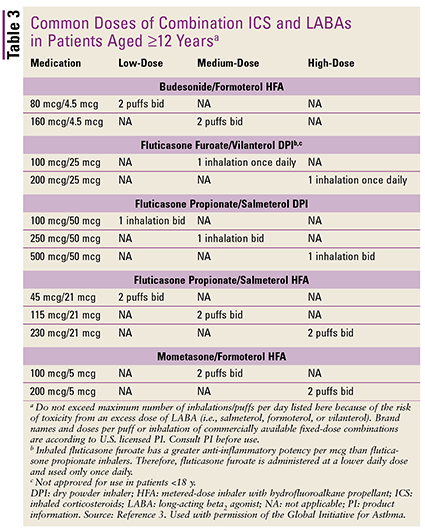
Step 4 involves a dose increase of the ICS/LABA combination to medium or high.2 If this strategy is ineffective, other controller options include increasing to a medium/high ICS plus LTRA or theophylline and adding tiotropium. However, tiotropium is indicated only for patients older than 18 years, and more clinical evaluation is needed before widespread recommendation of tiotropium as an alternative to LABA.10
LABAs work by the same mechanism as SABAs: A cascade effect causes a reduction in intracellular calcium, resulting in relaxation of smooth muscle in the airways. The pharmacist should be aware that asthma deaths increase with LABA use and that LABA administration without a long-term asthma-control medication is contraindicated.11
Step 5 warrants referral to a specialist and the recommendation to include an anti-immunoglobulin E (IgE), an anti-interleukin-5 (IL-5), or tiotropium.3 The anti-IgE omalizumab is approved for asthma prevention in patients aged older than 6 years. Omalizumab is administered by SC injection every 2 to 4 weeks and is adjusted by body weight and total serum IgE levels; doses exceeding 150 mg should be administered at multiple sites.12 The anti–IL-5 mepolizumab is approved for use in patients aged older than age 12 years to prevent asthma symptoms and is administered every 4 weeks by SC injection.13 Another recommendation at this stage is the addition of an oral corticosteroid, such as prednisone or dexamethasone.3
The pharmacist must be able to determine whether an asthma exacerbation needs emergent care. Transfer to an acute-care facility is warranted when the patient is not speaking in complete sentences, sits hunched forward, has a pulse rate exceeding 120 bpm, exhibits a respiratory rate greater than 30 breaths per minute, is drowsy or confused, or has a silent chest. Prior to the transfer, four to 10 puffs of a SABA, oxygen, and a systemic corticosteroid should be administered. This is also the correct course of action if the patient is treated for a mild exacerbation but does not improve after 1 hour of treatment with four to 10 puffs of an SABA every 20 minutes.3
Children Aged Less Than 5 Years
GINA’s stepwise approach for asthma treatment in children aged less than 5 years is similar to that presented in FIGURE 1, but with some differences.
Step 1 consists of SABA treatment alone for acute episodes without consideration of low-dose ICS. Patient progress is reassessed 3 months after treatment initiation. This step is reserved for patients with viral symptoms who otherwise have few wheezing episodes.3 As with adults, the addition of ipratropium to albuterol has shown better results than albuterol alone for treatment of acute exacerbations; however, the combination has not been shown to reduce hospital stays associated with asthma in children less than 5 years.3
Step 2 pertains to patients with persistent asthma episodes or three or more exacerbations per year. It consists of daily low-dose ICS along with the option of montelukast. ICS for children aged 5 years and younger are beclomethasone dipropionate, nebulized budesonide, fluticasone propionate, and mometasone furoate. Budesonide pressurized metered-dose inhaler (pMDI), ciclesonide, and triamcinolone acetonide have not been studied in young children. If this approach is ineffective, intermittent ICS are an approved option.3 Theophylline is not recommended.
Step 3 is based on a confirmed diagnosis of asthma. The patient’s caregiver should be instructed to evaluate proper technique, treatment adherence, and exposure to allergens. If these criteria are satisfied, the recommended course of action is to double the ICS dose from Step 2 and consider adding montelukast.3
Step 4 involves ensuring that the Step 3 criteria have been satisfied. If so, controller medications are continued and a referral to a specialist is given.3
For children aged 0 to 3 years, the preferred method of administering asthma medications is to use an MDI plus a spacer with a face mask. If the child is aged 4 to 5 years, a pMDI plus a spacer with a mouthpiece is preferred.3
Transfer to an emergency-care center is warranted by inability to speak or drink, bluish discoloration, stomach going below the rib cage during breathing, or a silent chest. Lack of response to albuterol treatment after 1 to 2 hours also calls for transfer to an emergency-care facility.
THE PHARMACIST’S ROLE
Regardless of the patient’s age, the pharmacist should be vigilant in educating the patient or caregiver. The patient must understand the difference between preventive and reliever medications and proper administration techniques. If the medication is equipped with a dose counter, the pharmacist should educate the patient on its application and instruct the patient to refill the inhaler before it is empty. The patient should be reminded to routinely clean inhalers and spacer devices.
The patient or caregiver should be asked to demonstrate use of the device (using a placebo inhaler) to ensure proper technique. If a spacer is not used, the pharmacist should verify that the patient first shakes the inhaler well, tilts the head back slightly, places the mouthpiece either in the mouth with lips tightly sealed around the opening or 1 to 2 inches away from the mouth, depresses the actuator when beginning to inhale, and holds the breath for approximately 5 seconds. If a spacer is used, it should be verified that the patient first shakes the inhaler, places the mouthpiece in the rubber opening of the spacer device, exhales, seals the lips tightly around the mouthpiece, depresses the actuator when beginning to inhale, and holds the breath for approximately 5 seconds. If a nebulizer is used, the pharmacist should ensure that the patient knows how to load the medication properly, seal the lips tightly around the mouthpiece, or (if using a mask) secure it around the nose and mouth. The importance of daily cleaning of nebulizer equipment should be emphasized.
Finally, the pharmacist should verify that the patient has a written action plan—customized to the needs of the patient—to manage asthma symptoms. The action plan should include a strategy to minimize exposure to irritants, recognize and treat worsening symptoms with the appropriate medication and technique, and address how to recognize, and proceed during, emergent events.
With proper counseling, monitoring, and management, the asthma patient can lead a healthy and enjoyable life.
REFERENCES
1. Hsu J, Sircar K, Herman E, Garbe P. EXHALE: A Technical Package to Control Asthma. Atlanta, GA: National Center for Environmental Health, CDC; 2018.
2. Sorkness CA, Blake KV. Asthma. In: DiPiro ST, Talbert RC, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill Education; 2017:344-347.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2018. www.ginasthma.org. Accessed April 18, 2018.
4. QVAR Redihaler (beclomethasone) package insert. Frazer, PA: Teva Respiratory, LLC; March 2018.
5. AccuNeb (albuterol) package insert. Morgantown, WV: Mylan Pharmaceuticals; January 2013.
6. Ventolin (albuterol) package insert. Research Triangle Park, NC: GlaxoSmithKline; May 2017.
7. Theophylline package insert. Princeton, NJ: Bristol-Myers Squibb; November 2006.
8. Singulair (montelukast) package insert. Whitehouse Station, NJ: Merck & Co, Inc; March 2018.
9. Accolate (zafirlukast) package insert. Tallahassee, FL: IPR Pharmaceuticals; May 2010.
10. Wenzel S. Treatment of severe asthma in adults and adolescents. UpToDate. Waltham, MA: UpToDate; 2018.
11. Formoterol package insert. Whitehouse Station, NJ: Merck & Co, Inc; November 2012.
12. Xolair (omalizumab) package insert. East Hanover, NJ: Genentech USA, Inc; May 2018.
13. Nucala (mepolizumab) package insert. Philadelphia, PA: GlaxoSmithKline LLC; December 2017.
14. Xopenex (levalbuterol) package insert. Marlborough, MA: Sepracor Inc; December 2009.
15. Xopenex HFA (levalbuterol) package insert. Marlborough, MA: Sunovion Pharmaceuticals Inc; March 2017.
To comment on this article, contact rdavidson@uspharmacist.com.





