US Pharm. 2013;38(4):HS2-HS5.
ABSTRACT: Invasive aspergillosis is a lethal fungal infection that usually affects the lung cells of immunocompromised hosts. If left untreated, the disease can spread into other vital organs, including the brain and the heart. In recent years, the frequency of this fatal condition has increased significantly owing to greater numbers of patients with neutropenia, malignancies, organ transplants, or advanced AIDS. Antifungal agents continue to be the mainstay of treatment to reduce the fungal burden. Amphotericin B (AmB), a polyene antibiotic that previously was used to treat this condition, has produced variable responses and serious adverse effects. Current treatment options utilize different azole compounds, lipid formulations of AmB, and echinocandins. These newer broad-spectrum antifungals are well tolerated and produce desirable therapeutic outcomes.
The occurrence of invasive aspergillosis, a fatal systemic fungal infection, has increased drastically over the past few decades, and the death toll has risen by more than 300%.1 Invasive aspergillosis is predominantly caused by Aspergillus fumigatus, although other Aspergillus species, such as Aspergillus flavus, Aspergillus niger, and Aspergillus terreus, also may be involved.2
Invasive aspergillosis poses a serious health risk for severely immunocompromised patients, including those with neutropenia, hematologic malignancies or other disorders, transplants (organ and hematopoietic stem cell), and advanced AIDS.3 Individuals receiving cytotoxic chemotherapy or immunosuppressive agents are equally vulnerable to systemic fungal diseases. Patients with severe chronic obstructive pulmonary disease and critically ill patients (liver failure, burn injury, etc.) without the above risk factors frequently are vulnerable to invasive aspergillosis.3
Aspergillus spores usually enter the lower respiratory tract of the host via inhalation.3 Other areas where the infection can originate include the sinuses, gastrointestinal (GI) tract, and skin. Symptoms of infection include fever, cough, dyspnea, production of sputum, chest pain, and hemoptysis. Invasive aspergillosis can spread to other organs, including the brain, heart, and skin.3
Antifungal therapy remains the mainstay of treatment for invasive aspergillosis.2 Aggressive treatment with such agents is critical for removing the fungal burden from the host. Reversal of neutropenia and immunosuppression is advised.2 Depending upon the severity of the infection (e.g., pericardial involvement), surgical procedures may be necessary.
Therapeutically Important Antifungal Agents
See TABLE 1 for the mechanisms of these agents.
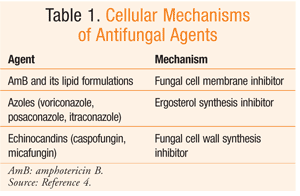
Polyene Antibiotic (Amphotericin B): Amphotericin B (AmB) is a polyene (containing multiple double bonds) macrolide antibiotic originally isolated from a Streptomyces species.4 It binds to ergosterol, a component of the fungal cell membrane. Binding to ergosterol destroys fungal membrane integrity, resulting in leakage of cellular content and then cell death. Being highly insoluble in water and in its original formulation, AmB was complexed with deoxycholate.4
AmB is poorly absorbed orally and requires parenteral administration.4 Following IV administration, the drug is released slowly and is highly bound to protein (>90%). AmB accumulates significantly in the liver and spleen, and it is present in synovial, pleural, and peritoneal fluid. Central nervous system (CNS) penetration is minimal. AmB has a half-life of 15 days and is excreted very slowly from the kidney.
AmB is associated with two major adverse effects (AEs): infusion-related reactions and renal toxicity. AmB-induced infusion reactions include fever, shaking, chills, hypotension, and tachypnea.4 Analgesics and corticosteroids are used to minimize these symptoms. AmB-induced renal toxicity is characterized by renal ischemia, hypokalemia, and tubular acidosis. AmB also reduces the production of erythropoietin (a glycoprotein that stimulates bone marrow to produce RBCs) in the kidney. AmB should not be administered concurrently with other nephrotoxic agents (aminoglycosides, nonsteroidal anti-inflammatory drugs). Serious AEs have prompted the development of lipid formulations of AmB. Lipid formulations that minimize the renal toxicity of AmB are AmB colloidal dispersion (ABCD), liposomal AmB (L-AmB), and AmB lipid complex (ABLC).4 These formulations have largely replaced AmB.
Azoles (Voriconazole, Posaconazole, Itraconazole): Chemically, azoles are triazole derivatives. These agents act by inhibiting fungal sterol-14-alpha-demethylase, a cytochrome-dependent enzyme associated with ergosterol synthesis. Inhibition of this enzyme enables the accumulation of methylsterol, thus impairing other cellular functions.4 Fungal cells acquire resistance to azoles by 1) increasing production of the target enzyme, 2) introducing mutations in the gene encoding sterol-14-alpha-demethylase, and 3) producing drug efflux proteins. As explained below, azoles can interfere with CYP-mediated metabolism of other drugs, resulting in drug-drug interactions and significant AEs.4
Voriconazole is formulated for oral and parenteral administration. Minimally bound to plasma proteins, it is metabolized in the liver by CYP enzymes (CYP3A4, CYP2C9, CYP2C19) and also inhibits these enzymes. Voriconazole is excreted renally. Transient visual disturbances (blurred vision, changes in color vision or brightness) are associated with this drug.4
Posaconazole is administered orally as a suspension. Its absorption is increased with food. Posaconazole is metabolized by glucuronidation and is excreted in the feces. The drug inhibits CYP3A4 and is a substrate of P-glycoprotein (PgP). AEs include headache and GI distress.4
Itraconazole is available in oral and parenteral formulations. It is absorbed well orally in the presence of food and low pH and is excreted fecally. Itraconazole is significantly bound to plasma proteins and is metabolized by CYP3A4. Hydroxyitraconazole is an active metabolite of the parent drug. Itraconazole inhibits CYP enzymes and PgP, and it results in liver toxicity. The drug has a negative inotropic effect on the heart and should not be used in patients with ventricular dysfunction or congestive heart failure.4
Echinocandins (Caspofungin, Micafungin): These agents inhibit 1,3-beta-glucan synthase, an enzyme responsible for fungal cell wall synthesis.4 Echinocandins have selective toxicity against fungi because mammalian cells do not possess a cell wall. Chemically, echinocandins are semisynthetic lipopeptides and are administered IV only. These agents are significantly bound to plasma proteins (>95%), and CNS penetration is inadequate. Echinocandins are excreted predominantly via the feces.4 Echinocandins do not induce or inhibit CYP enzymes, nor do they interact with PgP.4 As a result, the potential for drug interactions with other therapeutic agents is negligible. Echinocandins are usually well tolerated, and the most common AEs are elevated liver enzymes and creatinine (Cr), histamine-mediated effects (rash, pruritus, facial swelling), GI distress, headache, and pyrexia.
Treatment Recommendations
Without immediate clinical intervention, invasive aspergillosis can disseminate to other parts of the body (e.g., brain, major blood vessels, heart), causing more complications and poor outcomes.2 Pending diagnosis, antifungal treatment should be initiated immediately in patients with strongly suspected invasive aspergillosis. IV or oral voriconazole is the primary therapy for most patients with invasive aspergillosis.2 Parenteral voriconazole should be administered at a dosage of 6 mg/kg IV for day 1, followed by 4 mg/kg IV every 12 hours.2 Oral voriconazole may be administered at a dosage of 200 mg every 12 hours. For seriously ill patients, the parenteral formulation should be used. Primary alternative therapies including L-AmB should be considered. Salvage therapy options include ABLC, caspofungin, micafungin, posaconazole, and itraconazole (see TABLE 2 for dosages).
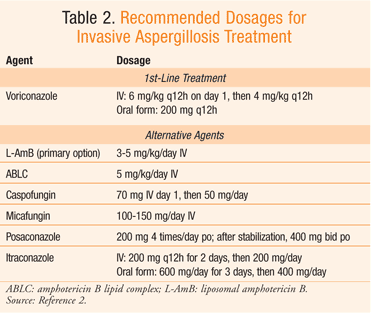
Combination drug therapy is not routinely recommended because of the lack of efficacy data.2 Incorporation of additional drugs into the current regimen or use of a drug combination comprising drug classes different from those in the original regimen is permissible for salvage therapy.2 Although the duration of antifungal therapy for invasive aspergillosis is poorly defined, treatment should be administered for at least 6 to 12 weeks.2 Surgical resection of fungus-infected tissue may be necessary in patients with lesions adjacent to major blood vessels, cardiac tissues, or the pleural space or ribs. Neutropenic patients with invasive aspergillosis who are not receiving colony-stimulating factor (CSF) may benefit from the addition of granulocyte CSF or granulocyte-macrophage CSF.2 Similarly, interferon-gamma may be useful as adjunctive therapy in neutropenic patients with chronic granulomatous disease (an inherited disorder of phagocytic cells).2
Important Clinical Trials
AmB: In the past, invasive aspergillosis cases were treated with AmB 1 to 1.5 mg/kg daily.3 A review of clinical data on 1,223 cases of invasive aspergillosis suggests that response rates (RRs) following AmB treatment for 14 or more days vary widely depending upon the underlying disease state.5 For example, the RR in heart and kidney transplant recipients with invasive pulmonary aspergillosis was high (83%), while a poor RR was reported in liver transplant patients (20%); the RR in leukemia patients ranked in between (54%). Respective RRs in patients with AIDS or bone marrow transplantation were 37% and 33%.5 Based on these results, clinical trials with lipid formulations of AmB were undertaken to improve therapeutic outcomes.
L-AmB: A large, multinational, double-blind study compared two dosages of L-AmB in patients with suspected or proven invasive fungal infections (invasive aspergillosis in approximately 96% of cases) and neutropenia at baseline.6 The study was carried out at 71 clinical sites in Europe and Australia. One group of patients (n = 107) received the standard L-AmB dosage of 3 mg/kg daily; the high-dose (HD) regimen of L-AmB 10 mg/kg daily was administered to another 94 patients. After 14 days, all patients received L-AmB 3 mg/kg daily until termination of treatment. The primary endpoint was favorable overall response (OR) (partial or complete) in HD versus standard-dose (SD) groups on day 14. Secondary endpoints included survival and safety outcomes.6 Favorable OR was 50% and 46%, respectively, in SD and HD groups (not significant). The survival rate (SR) at 12 weeks was 71% and 58%, respectively, for SD and HD groups.6 Nephrotoxicity (doubled serum Cr level at baseline) and grade 3 hypokalemia (serum potassium <3.0 mmol/L) were significantly higher in the HD group. The L-AmB 10 mg/kg daily dosage was not superior to the standard dose; rather, it increased the rate of toxic events.6
Voriconazole: A prospective, randomized trial compared the efficacy and toxicity of AmB and voriconazole in patients with immunocompromised conditions accompanied by definitive or probable invasive aspergillosis.7 Of 277 patients, 144 received voriconazole and the remaining 133 received AmB. Respective dosages for voriconazole and AmB were 4 mg/kg twice daily IV and 1 to 1.5 mg/kg daily IV. Following a minimum 7-day treatment period of parenteral voriconazole, the protocol permitted a switch to oral voriconazole 200 mg twice daily.7 The median duration of therapy for AmB and voriconazole, respectively, was 10 days and 77 days. Patients unresponsive or intolerant to the study drug were administered other licensed antifungal agents.
At week 12, a favorable outcome was observed in 53% of voriconazole patients, while in the AmB treatment arm a successful outcome was reported in 32% patients (absolute difference 21%). The 12-week SR was 71% in the voriconazole group and 58% in the AmB group.7 Voriconazole-treated patients had fewer severe drug-associated AEs. Nonetheless, transient visual disturbances (e.g., blurred vision, altered visual perception, altered color perception, and photophobia) were frequent in voriconazole-treated patients (45%).7
A follow-up analysis of this trial evaluated the impact of other licensed antifungal agents (for patients nonresponsive or intolerant to the study drug) on therapeutic outcomes.8 Fewer patients in the voriconazole group (36%) required other licensed antifungal agents, compared with patients in the AmB group (80%).8
Itraconazole: In an open trial, 21 immunocompromised patients with invasive aspergillosis refractory to AmB were treated with IV itraconazole 200 mg for 2 weeks (200 mg twice daily for days 1 and 2, then 200 mg once daily for 12 days), followed by oral itraconazole 200 mg twice daily for 12 weeks.9 Ten patients completed the entire course of treatment. Fifty-two percent of patients had a favorable outcome (complete or partial response). Total incidences of AEs were higher during the IV treatment period.9
In another study, 31 immunocompromised patients with invasive aspergillosis received successive treatment of IV itraconazole therapy for the first 2 weeks, followed by oral itraconazole for a median of 78 days.10 A positive outcome was reported in 15 patients (48%) at the last treatment evaluation, with eight patients achieving complete response.10
Caspofungin: In one study, 83 patients with proven invasive aspergillosis who were intolerant of or refractory to AmB, lipid formulations of AmB, and triazoles were treated with caspofungin (70 mg loading dose on day 1, then 50 mg daily for rest of treatment period).11 Patients were treated for at least 28 days, and another 7 to 14 days after symptom resolution. A positive RR of 45% was reported. Hepatic or renal toxicity from drug treatment was reported in fewer than 5% of patients.11
Conclusion
According to current treatment guidelines, IV or oral voriconazole is recommended for most patients with invasive aspergillosis.2 Patients refractory to or intolerant of voriconazole may be treated with L-AmB therapy as the primary alternative agent.2 Salvage therapy options for invasive aspergillosis include lipid formulations of AmB, posaconazole, itraconazole, caspofungin, or micafungin.2
In the future, opportunities will exist to evaluate combination antifungal therapy as a viable treatment option for invasive aspergillosis. Similarly, sequential treatment with drugs of different classes may be explored. Also, since clinical data on micafungin and anidulafungin for invasive aspergillosis are inadequate, robust clinical trials are needed to determine their efficacy and safety in Aspergillus infections.
REFERENCES
1. McNeil MM, Nash SL, Hajjeh RA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin Infect Dis. 2001;33:641-647.
2. Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of
aspergillosis: clinical practice guidelines of the Infectious Diseases
Society of America. Clin Infect Dis. 2008;46:327-360.
3. Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20:156-174.
4. Sheppard D, Lampiris HW. Antifungal agents. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York, NY: McGraw-Hill Medical; 2009:835-844.
5. Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608-615.
6. Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B
as initial therapy for invasive mold infection: a randomized trial
comparing a high-loading dose regimen with standard dosing (AmBiLoad
trial). Clin Infect Dis. 2007;44:1289-1297.
7. Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus
amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408-415.
8. Patterson TF, Boucher HW, Herbrecht R, et al. Strategy of
following voriconazole versus amphotericin B therapy with other licensed
antifungal therapy for primary treatment of invasive aspergillosis:
impact of other therapies on outcome. Clin Infect Dis. 2005;41:1448-1452.
9. Caillot D. Intravenous itraconazole followed by oral itraconazole
for the treatment of amphotericin-B-refractory invasive pulmonary
aspergillosis. Acta Haematol. 2003;109:111-118.
10. Caillot D, Bassaris H, McGeer A, et al. Intravenous itraconazole
followed by oral itraconazole in the treatment of invasive pulmonary
aspergillosis in patients with hematologic malignancies, chronic
granulomatous disease, or AIDS. Clin Infect Dis. 2001;33:e83-e90.
11. Maertens J, Raad I, Petrikkos G, et al. Efficacy and safety of
caspofungin for treatment of invasive aspergillosis in patients
refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39:1563-1571.
To comment on this article, contact rdavidson@uspharmacist.com.





