On March 23, 2010, the Obama Administration signed the Biologics Price Competition and Innovation (BPCI) Act of 2009. This legislation is a part of the health care reform (Patient Protection and Affordable Care Act) that created an abbreviated approval pathway for biologics that have proven to be highly similar (biosimilar) to or interchangeable with an FDA- approved biologic (reference product).1
Prior to the BPCI, applicants or sponsors could pursue a nonabbreviated Biologic License Application (BLA) under the Public Health Service (PHS) Act; or a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) under the Food, Drug, and Cosmetic Act (FDCA). The new legislation consolidates biologic regulation under one act, the PHS Act.1 Though this legislation alleviates controversy with regard to the regulatory framework concerning biologics, debates continue in areas such as the filing or application process, market exclusivity, and data exclusivity.
Biologics vs. Biosimilars
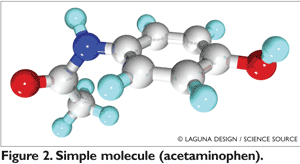
In the past, the initial controversy surrounding biosimilars had to do with the complexity of biologics’ molecules and the perception that exact duplication of such products could be challenging. Biologics are larger molecules and are generally produced using a living system or organism. They contain hundreds or even thousands of different atoms joined by long, winding chains (FIGURE 1). In contrast, chemical drugs are small molecules, very simple in size and characteristics (FIGURE 2). In addition, biologics are highly sensitive to the environmental condition of the production process, and minor manufacturing changes often alter the properties of the biologics.2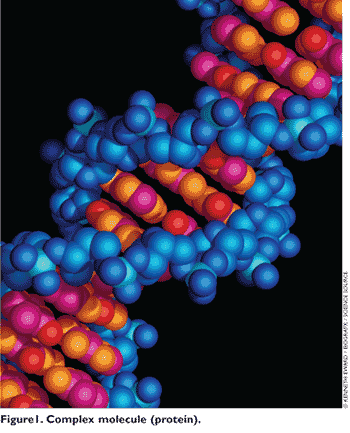
The Hatch-Waxman Act of 1984 established an abbreviated
pathway for the approval of generic chemical drugs regulated by the
FDCA. Section 505 (j) of the Hatch-Waxman Act established the ANDA, in
which generics manufacturers can seek FDA approval if their product’s
active ingredient, route of administration, dosage form, and strength
are the same as those of the reference product (
TABLE 1).
Manufacturers are generally not required to include animal
(preclinical) and human (clinical) investigations to establish safety
and effectiveness. Bioequivalence is defined as the relationship between the brand drug and the generic
drug.
Applicants must prove bioequivalence through testing that measures the
time it takes the generic drug to reach the bloodstream of healthy
volunteers in 24 to 36 hours. This time must be the same as that of the
reference product.3
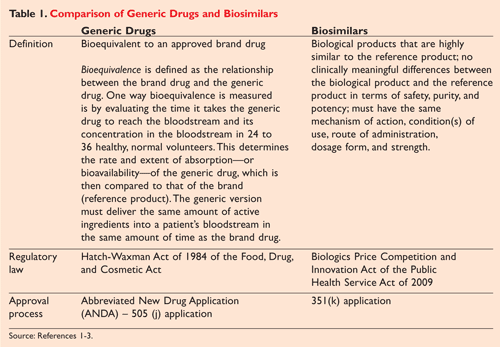
In contrast, under the BPCI a biological product that is deemed a biosimilar should be (1) highly similar to the reference product notwithstanding minor differences in the clinically inactive component, and (2) demonstrate no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product. A sponsor may seek approval of a biosimilar product under the 351(k) section of the PHS Act. Compliance with these criteria must be proven by analytical studies, animal studies including assessment of toxicity, or a clinical study. A clinical study or studies should be sufficient to demonstrate safety, purity, and potency in one or more appropriate conditions. The new legislation does allow the FDA the discretion to determine whether any of these studies, including the required clinical studies, are unnecessary for the application process.4
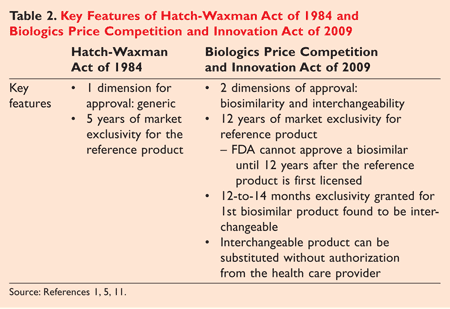
Under the BPCI, biologics are separated into two distinct categories: (1) products that are “biosimilar” to a reference product, and (2) products that are “interchangeable” with the reference product. In order to meet the higher standard of interchangeability, a sponsor must demonstrate that the
...biosimilar product can be expected to produce the same clinical result as the reference product in any given patient, and, for a biological that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.4
Other important distinctions between “biosimilar” products and “interchangeable” products relate to exclusivity (TABLE 2 ) . First, only biosimilar products that meet interchangeability criteria are eligible for market exclusivity for 12 years. If a sponsor uses the BLA pathway to bring to market a biologic, then market exclusivity does not apply. Moreover, the first sponsor to meet biosimilar requirements is not entitled to market exclusivity unless it obtains interchangeable status. Second, the new legislation provides the reference product with 12 years of data exclusivity. No sponsor can submit a biosimilar application until 4 years after the reference product is licensed. This is different from the Hatch-Waxman Act, which allows 5 years of data exclusivity.5
FDA Guidance
After the BPCI was enacted in 2010, the FDA was charged with clarifying expectations and providing guidance to sponsors looking to initiate biosimilar development programs. In February 2012, the FDA released three draft guidelines that give potential sponsors insight into the FDA’s current thinking on key scientific and regulatory factors involved in the submission of a 351(k) biosimilar application. The drafts reinforce the FDA’s “totality of evidence” approach to biosimilars, which implies that there is “no one-size-fits-all assessment” in this abbreviated process. The FDA reports that it will evaluate the sponsor’s integration of various types of information in the development plan. 6
First draft guideline: “Scientific Considerations in Demonstrating Biosimilarity to a Reference Product” assists in describing the FDA’s approach to evaluating the data and information submitted in support of obtaining biosimilar status to the reference product. The FDA recommends that sponsors use a stepwise approach to demonstrating biosimilarity. The approach should begin with structural analysis that will characterize the proposed product and the reference product with state-of-the art technology. The next step would be functional assays to provide evidence that the pharmacologic activity and potency of the proposed product are highly similar to those of the reference product and/or to demonstrate that there are no clinically meaningful differences between the proposed product and the reference product. The PHS Act also requires that information be included in the application demonstrating biosimilarity based on data from animal studies (assessment of toxicity included) unless the FDA determines that such studies are not necessary. The clinical program of the 351(k) application must include a clinical study or studies (including an assessment of immunogenicity, pharmacokinetics, and pharmacodynamics) “sufficient to demonstrate safety, purity, and potency in one or more conditions of use for which the reference product is licensed and intended to be used and for which licensure is sought for the biological product.” The FDA reports that the scope and magnitude of clinical studies will depend on the extent of the structural and functional assays and possible animal studies.7
Second draft guideline: “Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product” provides guidance on analytical studies that may be relevant to assessing whether the proposed protein product and the reference product are highly similar. The FDA suggests that during the assessment, the manufacturers should consider including the following components: expression system, manufacturing process, assessment of physiochemical properties, functional activities, receptor binding and immunochemical properties, impurities, and stability. This document does not deal with guidelines for interchangeability with a reference product because this information is still under consideration.8
Third draft guideline: “ Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009” provides answers to common questions from persons interested in developing biosimilar products. The guideline addresses queries such as how to request meetings with the FDA, addressing differences in formulation from the reference product, and the standing of the FDA and biosimilars in pediatric patients.9
Conclusion
The status of sponsor proposals for biosimilars was reported during the FDA’s Biosimilar Guidance Webinar held in 2012. Thirty-five pre–Investigational New Drug (IND) meeting requests were presented for 11 reference products; 21 pre-IND sponsor meetings were held and 9 INDs were received.6
Although companies are already taking advantage of the new guidelines, the United States is behind in the biosimilar market. While the recent enactment of the BPCI is certainly a step forward for biosimilars, the U. S. is still many years behind Europe regarding actual implementation of a regulatory approval. As of 2012, 14 biosimilars had been approved in the European Union.6
The FDA is just in the beginning stages of the biosimilar journey, and implementation of an approval process could be in the distant future. However, it is noteworthy that the global market for biosimilars is estimated to reach $2 to $3 billion by 2015 and will be concentrated mostly in Europe.10 Thus, the ability to work fervently toward and ultimately achieve regulatory approval of a biosimilar pathway in the U.S. is likely to have a notable impact on the biologics industry.
REFERENCES
1. Bonilla J., Beaver N. The new US biosimilar legislation, one year later. BioProcess International. 2011: 22-30.
2. Arizona Bioindustry Association. Small molecules, large biologics and the biosimilar debate. www.bio.org/articles/how-do-drugs-and-biologics-differ. Accessed May 23, 2013.
3. The U.S. Food and Drug Administration. Abbreviated new drug application (ANDA): Generics. www.fda.gov/Drugs/Development ApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/default.html. Accessed March 25, 2013.
4. The U.S. Food and Drug Administration. Implementation of the Biologics Price Competition and Innovation Act of 2009. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/ucm215089.html. Accessed March 18, 2013.
5. The National Law Review. New U.S. law establishes long awaited abbreviated approval pathway for biosimilar. www.natlawreview.com/article/new-us-law-establishes-long-awaited-abbreviated-approval-pathway-biosimilars. Accessed March 18, 2013.
6. U.S. Food and Drug Administration. biosimilar guidance webinar, February 15, 2012. https://collaboration.fda.gov/p13473376/?launcher=false&fcsContent=true&pbMode=normal. Accessed March 15, 2013.
7. U.S. Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf. Accessed March 15, 2013.
8. U.S. Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity to a reference protein product. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134. Accessed March 23, 2013.
9. U.S. Food and Drug Administration. Guidance for industry: biosimilars: questions and answers regarding implementation of the Biologics Price Competition and Innovation Act of 2009. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM273001.pdf. Accessed March 23, 2013.
10. U.S. Food and Drug Administration; Fitch: FDA proposed guidelines open the door to biosimilars. Marketing Weekly News. March 3, 2012:536. http://search.proquest.com.proxy.pba.edu/docview/922857871?accountid=26397. Accessed May 23, 2013.
11. National Association of Boards of Pharmacy. Paving approval pathway for biosimilars presents unique challenges for FDA. www.nabp.net/news/paving-approval-pathway-for-biosimilars-presents-unique-challenges-for-fda/. Accessed May 23, 2013.
To comment on this article, contact rdavidson@uspharmacist.com.






