US Pharm. 2011;36(5)(Diabetes suppl):10-15.
The pathophysiology of type 2 diabetes mellitus (T2DM) involves a multitude of complex and interrelated mechanisms. Classically, three metabolic alterations have been identified that contribute to the development of T2DM: 1) insulin resistance, 2) an eventual decrease in insulin secretion from pancreatic beta cells, and 3) an excess production of glucose from the liver.1 More recently, additional pathophysiologic mechanisms have been identified that are implicated in contributing to impaired glucose homeostasis and the varied metabolic alterations associated with T2DM. These include a relative or absolute increase in glucagon secretion from alpha cells of the pancreas, elevated serum free fatty-acid concentrations, an impaired incretin effect (e.g., decreased secretion of glucagon-like peptide-1 [GLP-1]), enhanced renal reabsorption of glucose, and dysregulation of appetite control via decreased neurotransmitter response in the brain.2 Thus, there is a tremendous amount of research under way to further elucidate these mechanisms and, more important, to discover pharmacologically based strategies that correct these separate, but intertwined, physiologic derangements.
The Evolving Diabetes Treatment Landscape
The current American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) approved algorithm for the treatment of T2DM is illustrated in FIGURE 1.3 Note that this consensus algorithm is a guide and that the clinical management of T2DM should always incorporate the patient’s unique goals, comorbidities, individual response to therapy, and other factors that may introduce unwanted drug interactions or contraindications to a particular therapy or therapies.
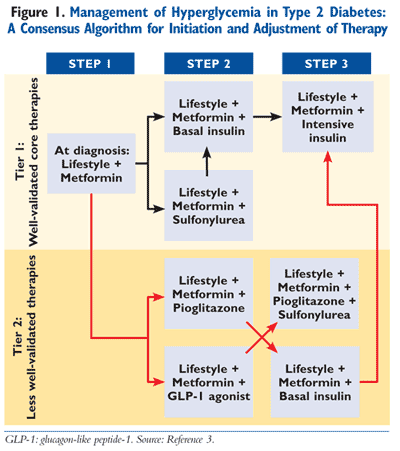
In contrast to the ADA/EASD algorithm, the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) algorithm divides treatment into three sections based on the hemoglobin A1C (A1C) level of the patient (6.5%-7.5%, 7.6%-9.0%, and >9.0%).4 All three levels of A1C control incorporate lifestyle modification as their starting point and base. For patients with an A1C of 6.5% to 7.5%, metformin is the preferred initial agent, with GLP-1 receptor agonists, dipeptidyl-peptidase-4 (DPP-4) inhibitors, thiazolidinediones (TZDs), or an alpha-glucosidase inhibitor listed as viable alternatives.4 Therefore, the use of a GLP-1 receptor agonist (exenatide, liraglutide) can be used as monotherapy or dual therapy in those with an A1C of 6.5% to 7.5%. (Exenatide is FDA approved as monotherapy for the management of T2DM.) Explicitly stated in the guideline for patients requiring dual or triple therapy, the AACE/ACE preferentially recommends the addition of a GLP-1 receptor agonist or a DPP-4 inhibitor (sitagliptin, saxagliptin), citing their efficacy and safety profiles as preferred agents over TZDs or sulfonylureas (SUs).4 The second A1C tier between 7.6% and 9.0% lists the use of a GLP-1 analogue or metformin as initial treatment or later as a component of triple therapy. For those with an A1C >9.0% who are drug naïve and without symptoms, once again a GLP-1 receptor agonist is recommended along with metformin.4
GLP-1 agonist therapy is an evolving and more acceptable treatment for T2DM regardless of the degree of A1C control. Therefore, the pharmacist must be prepared to explain the appropriate and safe use of GLP-1 receptor agonist therapy for patients being prescribed this class to meet target A1C goals. This article will review the role of GLP-1 receptor agonists in the treatment of T2DM with a focus on the safe and appropriate use of GLP-1 analogue therapies in those with T2DM, and the instrumental role pharmacists have in ensuring that patients are adequately educated regarding these agents.
Incretin Hormones
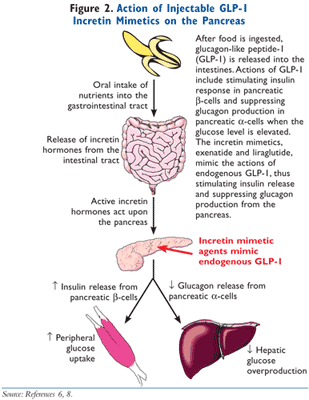
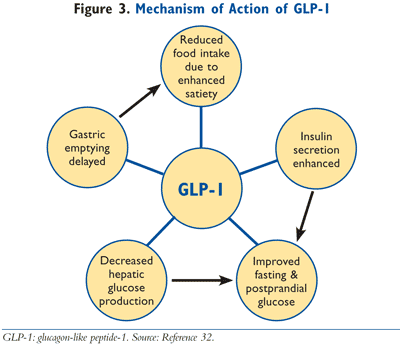
Orally ingested glucose stimulates insulin secretion to a greater degree than intravenously administered glucose, a phenomenon labeled the incretin effect.5 At one time, it was theorized that there was a single hormone responsible for this activity, but it was discovered that two hormones secreted from the intestines are primarily responsible for the incretin effect, GLP-1 and glucose-dependent insulinotropic polypeptide (GIP).6 These hormones are secreted into the intestines in response to the ingestion of a meal. The word incretin is derived from INtestinal seCRETion of INsulin. The GLP-1 receptor agonists, exenatide and liraglutide, mimic the action of endogenous GLP-1. As demonstrated in FIGURES 2 and 3, GLP-1 has multiple actions in the body. Of clinical significance, the incretin effect has been shown to be impaired in patients with T2DM, as GLP-1 secretion is deficient in these patients.7 In patients with T2DM, the release of incretin hormones is physiologically insufficient, thereby compromising the ability to adequately secrete insulin and also execute other physiologic processes required to meet the metabolic requirements to maintain normoglycemia.7,8 In summary, the incretin mimetics exert actions to normalize blood glucose levels via multiple actions in the face of declining endogenous GLP-1 levels.
Exenatide
Exenatide (Byetta), an incretin mimetic, is FDA approved for use as adjunctive therapy in patients whose T2DM is inadequately controlled with metformin, an SU, a TZD, or with combination of metformin plus an SU or TZD.9 Furthermore, exenatide is FDA approved to be used as monotherapy along with appropriate diet and exercise modifications.10 As shown in FIGURE 3, exenatide normalizes blood glucose levels by reducing fasting and postprandial glucose concentrations through a variety of mechanisms, including enhanced glucose-dependent insulin secretion, regulation of glucagon secretion, delayed gastric emptying, and decreased food intake due to centrally mediated effects on satiety.11
In clinical trials, exenatide has demonstrated efficacy in terms of both improved glycemic control and appetite suppression leading to weight loss.12-14 A1C reductions of 0.4% to 0.9% were observed in studies of exenatide, with higher reductions achieved at the 10 mcg twice-daily dose. Weight reductions were in the range of 0.9 to 2.8 kg over 30 weeks of treatment.12-14
In phase III clinical studies in which exenatide was administered concomitantly with metformin, an SU, or metformin plus an SU, 48% of the 483 patients receiving exenatide 10 mcg twice daily reported nausea compared to 18% of the 483 patients receiving placebo.12-14 Exenatide is available in pen form, potentially making dosing and administration more convenient (TABLE 1).

Exenatide is eliminated primarily through the kidneys, and its use is not recommended in patients with a creatinine clearance (CrCl) of <30 mL/min; however, no dose adjustment is required in those with a CrCl >30 mL/min.15 Dose adjustment does not appear to be necessary in patients with hepatic impairment, although exenatide has not been specifically studied in this population.11 Exenatide has been associated with hypoglycemia when given in combination with metformin, SUs, and TZDs.9 To avoid hypoglycemic events when dispensing exenatide in combination with an SU, it is important to reduce the SU dose on initiation of exenatide and when titrating the exenatide dose upward.10 When coadministered with a TZD, exenatide was associated with an 11% increased risk of hypoglycemia, compared with 7% for placebo.16
Safety of Exenatide: Over 30 cases of hemorrhagic or necrotizing pancreatitis associated with exenatide therapy have been reported to the FDA since the drug became available in June 2005.17 Therefore, physicians and pharmacists are encouraged to advise their patients taking exenatide to be aware of the signs and symptoms of acute pancreatitis and to seek prompt medical attention should they experience unexplained, persistent, severe abdominal pain, which may be accompanied by nausea, vomiting, and fever. If pancreatitis is suspected, the patient should discontinue exenatide immediately and seek medical attention. Information regarding the risk of acute pancreatitis was added to the U.S. labeling for exenatide in 2007.10 Pharmacists who are aware of cases of exenatide-associated acute pancreatitis or other serious adverse drug reactions are encouraged to report their findings to MedWatch, the FDA’s adverse drug reporting program (www.fda.gov/Safety/MedWatch/
Anti-exenatide Antibodies: In phase III clinical trials, 41% to 49% of patients receiving exenatide developed anti-exenatide antibodies.12-14 Titers tended to be low at week 30 and did not predict response to therapy or incidence of side effects or other safety issues. The clinical significance of anti-exenatide antibody formation is unknown at this time.
Weight Loss: In phase III trials, weight loss with the use of exenatide averaged between 0.9 and 2.8 kg over a 30-month time frame, with more weight loss associated when exenatide is added to existing metformin therapy.12-14 As many patients with T2DM are overweight or obese, a drug that can help patients achieve any amount of weight loss is often welcomed by patients and physicians alike. Furthermore, many drugs used to treat diabetes, such as SUs, TZDs, and insulin, are associated with weight gain; therefore, patients and health care providers are often searching for additional strategies to promote weight loss, and certainly strategies to avoid or attenuate weight gain.
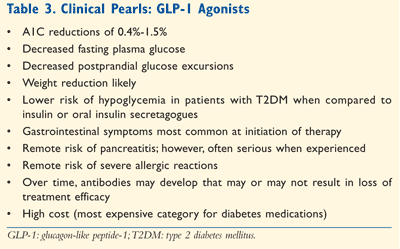
Counseling Patients on Exenatide: For patients with diabetes, reinforcing the importance of medical nutrition therapy (MNT) and the role of regular physical activity (exercise) cannot be revisited enough. Having pharmacists emphasize the importance of these approaches is paramount in assisting patients to achieve their A1C, lipid, and blood pressure goals. See TABLES 2 and 3 for additional counseling points for patients prescribed GLP-1 therapy.

Liraglutide
The injectable GLP-1 analogue, liraglutide (Victoza), is FDA approved for once-daily subcutaneous administration in combination with diet and exercise for the treatment of patients with T2DM.18 Liraglutide has been one of the most intensely studied diabetes therapies, with six phase III Liraglutide Effects and Action in Diabetes clinical studies (known as the LEAD trials) being performed prior to its FDA approval.19-24 Liraglutide has been studied as monotherapy and in combination with metformin, the SU glimepiride, the TZD rosiglitazone, or insulin glargine, and in combination with glimepiride and rosiglitazone, or metformin and an SU. Once-daily liraglutide compared favorably with twice-daily exenatide, with similar improvements in A1C and body weight reductions.
The most common adverse events associated with liraglutide therapy are dose-dependent nausea, vomiting, and diarrhea. Specifically in regard to nausea, it tended to be most pronounced in the first 4 weeks of therapy in all phase III LEAD trials, with symptoms generally dissipating over the remainder of the study period.19-24 As with exenatide, there is minimal risk of hypoglycemia when used with nonsecretagogue medications.
Results from a study examining the safety of using liraglutide in patients with moderate to severe renal impairment demonstrated no deleterious effects on serum creatinine (SCr) or increased incidence or severity of adverse events.25 Liraglutide was evaluated in 24 subjects with mild, moderate, severe, or no hepatic impairment.26 Subjects were administered 0.75 mg of liraglutide as a single dose, and evaluated after a 72-hour period to determine if hepatic impairment influenced liraglutide’s pharmacokinetic and safety profile. After both renal and hepatic evaluations, it was concluded that no hepatic or renal dosing adjustments are necessary with liraglutide.27 This is in contrast to exenatide, which is primarily eliminated through the kidneys, and is not recommended for use in patients with severe renal impairment or in those with end-stage renal disease (ESRD).10
Safety of Liraglutide: Pancreatitis has rarely been experienced by patients taking liraglutide (7 cases in phase II and III trials of 4,257 patients), prompting the close monitoring of the development of pancreatitis in patients prescribed liraglutide.28 The LEAD trials indicate liraglutide to be generally safe; however, most phase III clinical studies are of insufficient duration and do not include enough study participants to effectively gauge long-term safety. The development of rare adverse events, which may not occur until a patient has been on a drug for a number of years, is of concern, but until more patients are followed for an adequate period of time, the true safety of a drug cannot be stated or concluded. The safety of liraglutide (and exenatide) can only truly be assessed with robust phase IV postmarketing data involving long-term treatment with this novel therapy.
Animal studies involving liraglutide have been associated with the development of thyroid C-cell tumors; therefore, diligent postmarketing surveillance to elucidate the risk of medullary thyroid carcinoma (MTC) is ongoing. Liraglutide’s prescribing information contains a warning regarding the observation of dose-dependent and treatment-duration-dependent thyroid C-cell tumors witnessed at clinically relevant exposures in rats and mice.18
During the LEAD clinical trials, calcitonin, a biomarker for the detection of medullary thyroid cancer, was monitored routinely.28 While remaining within the normal range, increases in calcitonin levels did occur in a slightly higher percentage of patients treated with liraglutide in the clinical trials when compared to controls.28
Currently, the clinical and safety implications of these alterations in calcitonin levels are unknown. It is important for pharmacists to recognize that liraglutide is contraindicated in patients with a personal or family history of MTC and in patients with multiple endocrine neoplasia syndrome type 2 (MEN 2).18 Therefore, the FDA has requested the establishment of a cancer registry to monitor the annual incidence of MTC over the next 15 years.29
Antiliraglutide Antibodies: In three of the six phase III LEAD trials, anti-liraglutide antibodies were reported and developed as follows: in 9% to 13% of subjects receiving 0.6 mg to 1.8 mg (LEAD-1);19 in 4.1% of patients receiving 1.2 mg and 6.7% of patients receiving 1.8 mg (LEAD-4);22 and in 9.8% of subjects receiving 1.8 mg/day (LEAD-5).23 While the clinical and safety impact of antiliraglutide antibodies is unknown at this time, further study of this phenomenon is warranted.
Weight Loss: Average weight loss in the LEAD trials was 2 kg to 3.24 kg, with the majority of the reduction noted in the first 4 months of therapy, but tending to be sustained throughout the remaining period of the studies.12,19-24 Weight loss was due to reductions in fat mass rather than lean tissue mass.30 Furthermore, a comparative trial of exenatide and liraglutide in T2DM subjects concluded that both incretin mimetics were associated with a significant reduction in body weight when compared to baseline (2.87 kg and 3.24 kg, respectively).12
Of particular interest, a 20-week research trial involving nondiabetic patients with a mean body mass index of 30 to 40 kg/m2 at baseline compared the weight loss effects of liraglutide versus orlistat 120 mg three times daily or placebo.31 Liraglutide subjects lost 4.8 kg in the group receiving 1.2 mg and 5.5 kg in the 1.8 mg group when compared to baseline. This trial also studied a cohort receiving either 2.4 mg of liraglutide or 3.0 mg of liraglutide, with reported weight reductions of 6.3 kg and 7.2 kg, respectively, when compared to baseline. Those receiving orlistat experienced an average weight loss of 4.1 kg, and those receiving placebo lost 2.8 kg. Further research is under way to characterize the role of incretin mimetics in overweight or obese patients without diabetes.
Counseling Patients on Liraglutide: Exenatide involves only one dose titration, while liraglutide can involve up to two. It is very important that pharmacists effectively counsel patients on titration schedules and recognize and support the treatment expectations and goals of the patient. Liraglutide is recommended at a starting dose of 0.6 mg daily for the first week, followed by an upward titration to 1.2 mg daily.18 For those patients not achieving desired glycemic control at 1.2 mg, the dose can be increased to 1.8 mg. Per the prescribing information, it is also recommended that a reduction in the dose of pre-existing insulin secretagogue medications be considered when initiating liraglutide to minimize the risk of treatment-emergent hypoglycemia.18

Discussion
The pharmacokinetic profile of liraglutide is amenable to once-daily dosing, thus creating a potential advantage when compared to twice-daily exenatide. Drug regimen simplicity is an important clinical consideration, particularly in patients receiving multiple medications for the treatment of T2DM and related comorbidities. Patients often present with resistance to the initiation of an injectable agent; however, the potential for weight loss with the GLP-1 incretin mimetics can be a motivator for some patients. The most recent consensus algorithm released by the ADA/EASD lists GLP-1 analogues as a treatment option for consideration as a Tier 2 agent, or “less well-validated therapy,” in T2DM patients (FIGURE 1).3
The consensus guidelines recommend consideration of GLP-1 receptor agonist therapy in selected clinical situations. One situation in which GLP-1 receptor agonist therapy could be considered is when weight loss is a major consideration and the patient’s A1C level is close to target (<8.0%). The guidelines warn, however, that GLP-1 receptor agonist therapy is not indicated for all patients and should be used with caution in patients with a history of significant gastrointestinal disease, such as a diagnosis of gastroparesis, due to a possible exacerbation of the condition with incretin mimetic therapy.3 Because postprandial hyperglycemia affects A1C to a greater degree than fasting hyperglycemia, the closer a patient is to the A1C goal, GLP-1 receptor agonists provide a viable treatment option to target postprandial glucose excursions due to their glucose-dependent effects on insulin secretion.
Conclusion
Clinical trial data from large, controlled studies demonstrate the efficacy and safety of GLP-1 agonists in terms of A1C reduction, beneficial effects on body weight, and a low risk for hypoglycemic events when used as monotherapy. Both exenatide and liraglutide are relatively well tolerated, with dose-dependent nausea, vomiting, and diarrhea being the most commonly reported adverse events observed in clinical trials. Clinical trial data in humans indicate that exenatide or liraglutide may have a role in the treatment of T2DM as monotherapy or in addition to oral antidiabetic drugs. Questions remain about the safety of these drugs with regard to the risk of pancreatitis and the development of MTC in patients receiving liraglutide. Pharmacists play a critical role in educating patients on the appropriate and safe use of GLP-1 agonists and also bear responsibility in reporting suspected serious adverse events with this new class of antidiabetic agents.
REFERENCES
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Position statement. Diabetes Care. 2011;34(suppl 1):S62-S69.
2. DeFronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795.
3. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32:193-203.
4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540-559.
5. Elrick H, Stimmler L, Hlad CJ, et al. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082.
6. Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665-670.
7. Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609-613.
8. Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47:357-366.
9. Barnett A. Exenatide. Expert Opin Pharmacother. 2007;8:2539-2608.
10. Byetta (exenatide) injection package insert. San Diego, CA: Amylin Pharmaceuticals, Inc; September 2010.
11. Odegard PS, Setter SM, Neumiller JJ. Considerations for the pharmacological treatment of diabetes in older adults. Diabetes Spectrum. 2007;20:239-247.
12. Buse JB, Henry RR, Han J, et al, for the Exenatide-113 Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
13. DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092-1100.
14. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091.
15. Linnebjerg H, Kothare PA, Park S, et al. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64:317-327.
16. Zinman B, Hoogwerf BJ, Dúran Garcia S, et al. The effect of adding exenatide to thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477-485.
17. Information for healthcare professionals: Exenatide (marketed as Byetta)–8/2008 update. www.fda.gov/Drugs/DrugSafety/
18. Victoza (liraglutide) injection package insert. Princeton, NJ: Novo Nordisk Inc; January 2010.
19. Marre M, Shaw J, Brandle M, et al, for the LEAD-1 SU Study Group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268-278.
20. Nauck M, Frid A, Hermansen K, et al, for LEAD-2 Metformin Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin in type 2 diabetes mellitus (LEAD-2 Met). Diabetes Care. 2009;32:84-90.
21. Garber A, Henry R, Ratner R, et al, for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomized, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
22. Zinman B, Gerich J, Buse JB, et al, for the LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes mellitus (LEAD-4 Met + TZD). Diabetes Care. 2009;52:2046-2055.
23. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulphonylurea therapy in type 2 diabetes mellitus: a randomized controlled trial (LEAD-5 met + SU). Diabetologia. 2009;52:2046-2055.
24. Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786-1791.
25. Jacobsen L, Hindsberger C, Robson R, et al. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898-895.
26. Flint A, Nazzal K, Jagielski P, et al. Influence of hepatic impairment on pharmacokinetics of the long-acting human GLP-1 analogue liraglutide. Diabetes. 2007;56:A145.
27. Neumiller JJ, Sonnett TE, Wood LD, et al. Pharmacology, efficacy and safety of liraglutide in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:215-226.
28. Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774-777.
29. Turner RC, Cull CA, Frighi V, et al; UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005-2012.
30. Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163-1172.
31. Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. 2009;374:1606-1616.
32. Neumiller JJ, Campbell RK. Liraglutide: a once-daily incretin mimetic for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2009;43:1433-1444.
To comment on this article, contact rdavidson@uspharmacist.com.





