US Pharm. 2015;40(8)(Pharm&Tech suppl):8-11.
ABSTRACT: Following the legalization of electronic prescribing for Schedule II through V controlled substances in the United States by the Drug Enforcement Administration in June 2010 and the emergence of Prescription Drug Monitoring Programs (PDMPs), each state has developed and continues to revise its own set of statutes and rules governing these technological advancements. PDMPs are also proving to be a useful tool in combating controlled-substance abuse. As the shift to electronic prescribing of controlled substances (EPCS) and PDMP use continues, it is important for pharmacists to be aware of the national laws outlining EPCS use and to stay abreast of the state laws regarding this topic.
Electronic prescribing, or e-prescribing, is the practice of electronically sending an accurate, error-free, and understandable prescription directly to a pharmacy from the point of care. According to the Centers for Medicare and Medicaid Services (CMS), e-prescribing is an important element in improving the quality of patient care.1 E-prescribing became a hot topic after the 2003 approval of the Medicare Modernization Act, which offered standards for appropriate implementation and use.2 In 2009, Medicare created an incentive program for provider practices that were using e-prescribing programs. E-prescribing was further expanded with the inclusion of controlled substances by the Drug Enforcement Administration (DEA) in June 2010. E-prescribing systems, in addition to transmitting orders accurately between prescribers, pharmacies, and healthcare plans, have the potential to help providers avoid prescribing errors, adhere to treatment guidelines, and monitor patients’ responses to treatment. It is estimated that, in 2014, 56% of physicians prescribed medications electronically.3
Controlled-substance fraud and abuse are on the rise. It is estimated that between 3% and 9% of drugs that are diverted for abuse are tied to fraud and forgery of paper prescriptions.4 According to the CDC, more than 2 million Americans abused prescription painkillers in 2013, and there were 16,235 deaths involving prescription opioids.5 The CMS suggests that the adoption of electronic prescribing of controlled substances (EPCS) will aid in combating these statistics. Despite the potential advantages and a 400% increase in EPCS in 2014, it is estimated that only 1.4% of controlled-substance providers across the United States are set up for EPCS, whereas 75% of pharmacies are ready to receive these prescriptions.3
Software Requirements in E-prescribing
The DEA Electronic Prescriptions for Controlled Substances rule allows practitioners to write prescriptions for controlled substances electronically and permits pharmacies to receive, dispense, and store these prescriptions. The rule does not mandate that prescribers use EPCS, and it does not require pharmacies to accept them for dispensing. As far as the DEA is concerned, prescribing practitioners are still able to write and manually sign prescriptions for Schedule II through V controlled substances and give oral prescriptions for Schedule III, IV, and V controlled substances, and pharmacies are still able to dispense based on these prescriptions. EPCS is allowed only if both the electronic prescription and the pharmacy’s software application meet specific DEA requirements, and it is subject to state-specific laws and regulations. EPCS software applications must be reviewed and certified by an approved certification body before providers can begin issuing e-prescriptions for controlled substances.6
Title 21 Code of Federal Regulations (CFR), Part 1311, Subpart 3 addresses the requirements that must be met in order to issue and process Schedule II, III, IV, and V controlled-substance prescriptions electronically. This CFR outlines practitioner responsibilities; requirements for obtaining an authentication credential and two-factor authentication; e-prescription application requirements; requirements for establishing logical access control; transmission requirements; pharmacy application requirements; internal audit trails; third-party audits or certifications; and recordkeeping.6
Before a pharmacy application is used to process controlled-substance prescriptions, it is the responsibility of the pharmacy to determine that the third-party auditor or certification organization has found that the pharmacy application accurately and consistently imports, stores, and displays the information required for prescriptions and the indication of signing, number of refills, and the practitioner’s digital signature, where applicable. If a pharmacist is required to make a notation on an e-prescription, it must be done electronically when filling the prescription and retained electronically in the prescription record or in linked files. The application must be able to limit annotation, alteration, or deletion of prescription information. In addition, the application must digitally sign and archive a prescription, as well as verify a prescriber’s digital signature, and it must read, store, and retrieve prescription information, including number of units or volume of drug dispensed, date dispensed, and name or initials of the person who dispensed the prescription. The pharmacy application must conduct internal audits, generate reports, back up controlled-substance prescription records daily, and retain all archived records electronically for at least 2 years.6
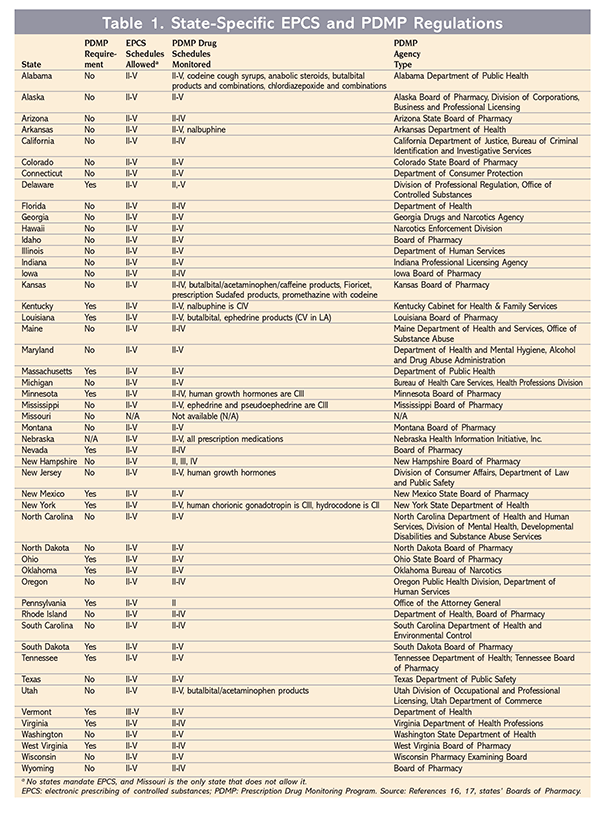
Practitioners who use EPCS must ensure, as with paper prescriptions, that the e-prescription conforms to the requirements of the Controlled Substances Act and DEA regulations. Practitioners sign e-prescriptions for controlled substances using a two-factor credential, which constitutes the legal signature of the DEA-registered prescribing practitioner. When prescribers use the credential, their application must digitally sign and archive the DEA-required information that is contained in the prescription.6 TABLE 1 lists state-specific information regarding EPCS, including monitoring requirements, governing regulatory agencies, and drug schedules allowed and monitored. (Missouri is the only state that does not allow EPCS, and no states mandate it.)
Impact of Monitoring Programs
President Obama’s drug-control priorities for the 2016 fiscal year include allocating funding to states that have adopted the use of Prescription Drug Monitoring Programs (PDMPs), another promising tool for combating prescription drug abuse. PDMPs can provide a prescriber or pharmacist with important information regarding a patient’s prescription history, allowing prescribers to identify patients who are potentially abusing medications.7 A PDMP is a statewide electronic database that collects designated data on substances dispensed in that state. The PDMP is housed by a specified statewide regulatory, administrative, or law-enforcement agency. Data from the databases are distributed to individuals who are authorized under state laws to receive the information as needed for their profession.8
Research suggests that PDMPs reduce the prescribing of Schedule II opioid analgesics, lower substance-abuse treatment admission rates, and result in lower annual increases in opioid misuse or abuse in states with PDMPs versus those without them.9-12 A study investigating the impact of PDMPs demonstrated that clinician review of PDMP data changed clinical management in 41% of the cases examined. The study revealed that 61% of these cases ended up receiving fewer or no opioids, while 39% received more opioid medication than previously planned, as the physician was able to confirm that the patient did not have a recent history of opioid abuse.13 Currently, 49 states, the District of Columbia, and one U.S. territory (Guam) have legislation authorizing the creation and operation of a PDMP, and all but the District of Columbia program are operational.7,14 PDMPs vary among states, including which state agencies house the PDMP, which controlled substances must be reported, how often data need to collected and reported, and who can access the PDMP. As states continue to enhance PDMPs to include collecting data for all controlled substances, proactive reporting to physicians and pharmacists, interstate data exchange, and integration with other health information technology systems to improve provider use, the effectiveness of PDMPs will continue to increase.7,15
As pharmacists move from an environment where e-prescribing is optional to one where it will likely become the norm or even required, they must familiarize themselves with the laws governing EPCS in their state. State-specific information can be found by visiting state Board of Pharmacy websites. Each state has varying rules and statutes regarding several important aspects of EPCS, such as state approval of e-prescribing vendors or transmission intermediaries, medications scheduled differently from the schedule set out by the DEA, requirements regarding review of PDMP databases before issuing new controlled prescriptions, handwritten prescriptions, and e-prescribing of CII substances versus CIII through V substances.
Conclusion
In summary, with controlled-substance fraud and abuse on the rise in the U.S., new and innovative techniques to combat these issues must be implemented. EPCS and PDMPs are relatively new tools that states are using to reduce prescription drug abuse. Despite the potential advantages of and a substantial increase in EPCS, only a small percentage of controlled-substance providers across the U.S. are set up to send controlled prescriptions electronically. There are currently 49 states with operational PDMPs; these PDMPs vary by state and will likely continue to be enhanced. Both EPCS and PDMPs will continue to evolve, so it is important for pharmacists to stay abreast of this topic by periodically visiting their state Board of Pharmacy website.
References
1. Centers for Medicare and Medicaid Services. E-prescribing. www.cms.gov/Medicare/E-Health/Eprescribing/index.html?redirect=/ePrescribing. Accessed May 19, 2015.
2. Medicare Prescription Drug, Improvement, and Modernization Act, Pub L No. 108-173, 117 Stat 2066 (2003).
3. 2014 national progress report. Arlington, VA: Surescripts; 2014.
4. 2013 national progress report and safe-Rx rankings. Arlington, VA: Surescripts; 2013.
5. CDC. Injury prevention & control: prescription drug overdose. www.cdc.gov/drugoverdose/data/overdose.html. Accessed May 30, 2015.
6. Office of Diversion Control. Title 21 Code of Federal Regulations. Part 1311—requirements for electronic orders and prescriptions. www.deadiversion.usdoj.gov/21cfr/cfr/1311/subpart_c100.htm#100. Accessed May 19, 2015.
7. Department of Health and Human Services. ASPE issue brief: opioid abuse in the U.S. and HHS actions to address opioid-drug related overdoses and deaths. http://aspe.hhs.gov/sp/reports/2015/OpioidInitiative/ib_OpioidInitiative.pdf. Accessed May 29, 2015.
8. National Alliance for Model State Drug Laws (NAMSDL). Prescription drug monitoring program: a brief overview. www.namsdl.org/library/1BB65CEB-1C23-D4F9-74870D15AD6B0D52. Accessed May 30, 2015.
9. Simeone R, Holland L. An evaluation of prescription drug monitoring programs. www.simeoneassociates.com/simeone3.pdf. Accessed July 13, 2015.
10. Curtis LH, Stoddard J, Radeva JI, et al. Geographic variation in the prescription of schedule II opioid analgesics among outpatients in the United States. Health Serv Res. 2006;41:837-855.
11. Reisman RM, Shenoy PJ, Atherly AJ, Flowers CR. Prescription opioid usage and abuse relationships: an evaluation of state prescription drug monitoring program efficacy. Subst Abuse. 2009;3:41-51.
12. Reifler L, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434-442.
13. Baehren DF, Marco CA, Droz DE, et al. A statewide prescription monitoring program affects emergency department prescribing behaviors. Ann Emerg Med. 2010;56:19-23.
14. Prescription Drug Monitoring Program Training and Technical Assistance Center. Prescription Drug Monitoring Program frequently asked questions. www.pdmpassist.org/content/prescription-drug-monitoring-frequently-asked-questions-faq. Accessed May 29, 2015.
15. Clark T, Eadie J, Knue P, et al. Prescription drug monitoring programs: an assessment of the evidence for best practices. www.pdmpexcellence.org/sites/all/pdfs/Brandeis_PDMP_Report.pdf. Accessed May 30, 2015.
16. Prescription Drug Monitoring Program Training and Technical Assistance Center. PDMP agency type. www.pdmpassist.org/content/pdmp-agency-type. Accessed May 30, 2015.
17. Prescription Drug Monitoring Program Training and Technical Assistance Center. Drug schedules monitored. www.pdmpassist.org/content/drug-schedules-monitored. Accessed May 30, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






