US Pharm. 2020;12(45):18-24.
ABSTRACT: Ulcerative colitis and Crohn’s disease, the two most common forms of inflammatory bowel disease (IBD), are inflammatory disorders of the digestive tract characterized by symptoms such as abdominal pain, nausea, and diarrhea. These symptoms can result in loss of appetite, reduced oral intake, and ultimately impaired nutritional status. Pharmacists have a unique opportunity to counsel patients on nutrition management in IBD while focusing on updated guidance that maximizes nutritional status, maintains adequate intake, and avoids foods that may exacerbate symptoms. Pharmacists can closely collaborate with their patients and IBD healthcare providers on nutritional intervention and education, as optimizing nutritional status is important in order to prevent long-term health consequences of malnutrition in this patient population.
Ulcerative colitis (UC) and Crohn’s disease (CD), the two most common forms of inflammatory bowel disease (IBD), are inflammatory disorders of the digestive tract characterized by symptoms such as abdominal pain, nausea, and diarrhea. These symptoms can result in loss of appetite, reduced oral intake, and ultimately impaired nutritional status.1
In 2015, approximately 1.3% (3 million) of U.S. adults reported receiving a diagnosis of IBD.1 This percentage is a sizable increase from 1999 (0.9%, or 2 million adults). With IBD incidence on the rise, measures to improve nutritional status and avoid food triggers have become increasingly important in the treatment of these patients. This is because malnutrition can occur in UC and CD. Malnutrition is a significantly greater issue in CD because of CD’s ability to affect any part of the gastrointestinal (GI) tract, whereas UC is limited to the colon and has few direct malabsorptive effects.
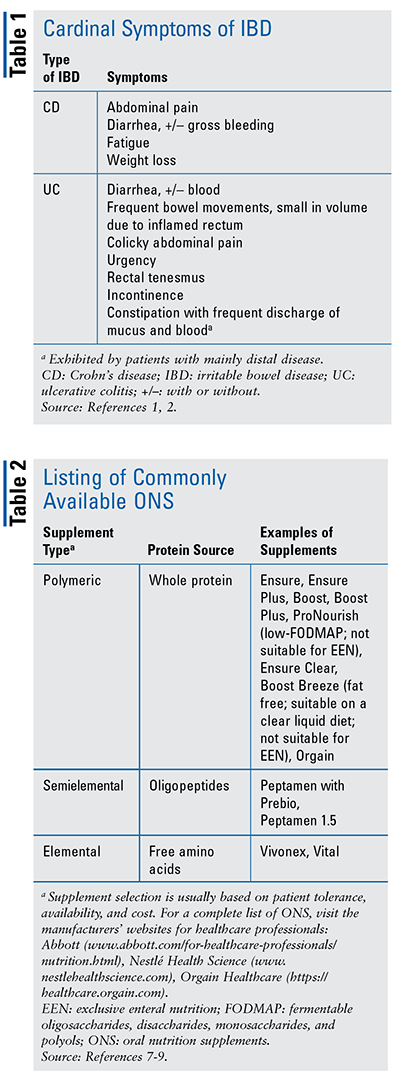
Because patients with IBD constitute a high-risk population for malnutrition, they need screening for malnutrition as well as subsequent assessment and management, all of which pharmacists can support to improve patient outcomes. By being aware of the cardinal symptoms that IBD patients exhibit (TABLE 1) early on, pharmacists can work with patients and their practitioners on nutritional care and implementation of updated guidelines, which can aid in identifying and thus preventing malnutrition and micronutrient deficiencies, weight loss, and osteoporosis, and (in children) can help promote optimal growth and development.1,2 This is important, as pharmacists may be the first point of contact for patients with initial symptoms or relapsing flares who are seeking counsel.3,4
Nutritional Support for Patients With Undernutrition
Enteral Nutrition (EN): EN is delivered to the digestive system in liquid form. Oral nutrition supplements (ONS) may be used to increase calorie and protein intake in patients who have undernutrition and active disease, but not as induction or maintenance therapy for IBD, so it is important to identify a patient’s disease course. Also, ONS may be used in patients with undernutrition who are in remission but cannot increase caloric intake through a standard diet.5,6
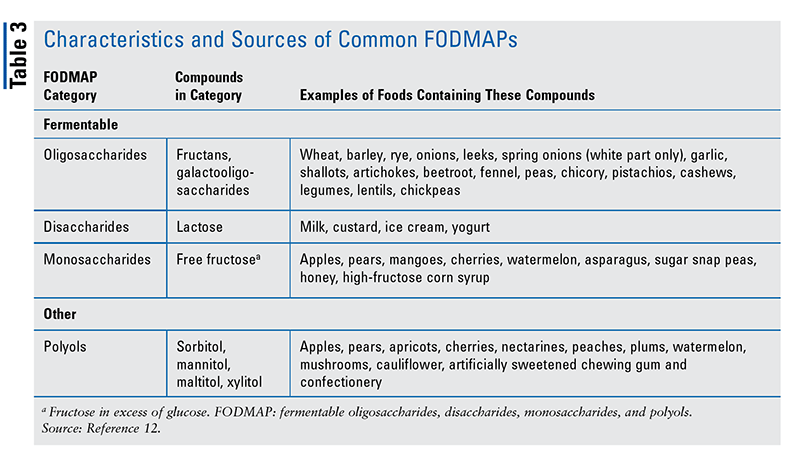
Selection of a specific type and quantity of EN is based on a patient’s calorie and protein requirements, and this is where multidisciplinary care between the physician, dietitian, and pharmacist is important to coordinate individualized nutrition plans for their patients with IBD. Liquid nutritional supplementation may take the form of a predigested (elemental or semielemental) or polymeric diet. Standard formulas are usually polymeric and tend to be the most palatable, whereas elemental or semielemental formulas may be better tolerated in patients with moderately to severely active IBD. Each type of formula consists of liquid nutrients in an easily assimilated form, with differing protein sources (TABLE 2).7-9 Additionally, pharmacists may review options with their patients in selecting specialty formulas free of common allergens (gluten, soy, dairy, corn, preservatives); one example is Orgain, which may contain simpler and no or limited artificial or synthetic chemicals.5,6
Parenteral Nutrition (PN): PN consists of administering calories, amino acids, electrolytes, vitamins, minerals, trace elements, and fluids via the IV route. In the setting of IBD, PN may be indicated for patients with short bowel syndrome or bowel obstruction, or for patients who are unable to eat or tolerate tube feedings. PN may also be used to correct nutritional deficiencies prior to surgery.10,11
Nutritional Support for Patients Without Undernutrition
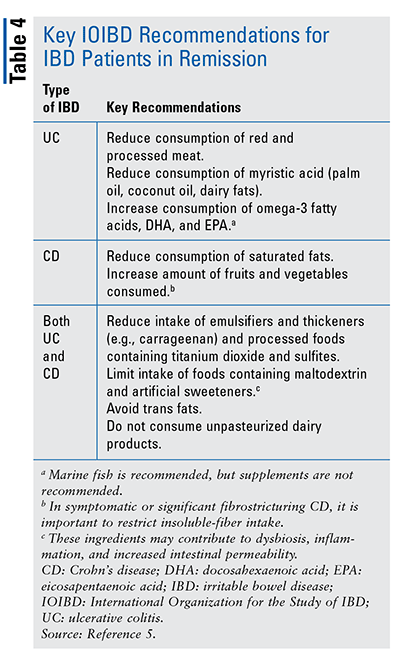
Patients With Active Disease: Pharmacists should impart to patients that, unfortunately, no particular food category or single food item is broadly associated with triggering a disease flare; nor is there an IBD diet that can be generally recommended to promote remission in IBD patients with active disease. That said, there are data that show patients with IBD achieving symptomatic improvement with certain interventions. Some patients may find relief from bloating or diarrhea by undertaking, for a period of time (approximately 2-6 weeks), a diet that is low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs; TABLE 3).12 Pharmacists can support patients who are working with their physician in following a low-FODMAP diet by helping them replace important nutrients that are temporarily excluded. For example, when a food item is eliminated, pharmacists can recommend alternative foods that supply the same nutrients and calories. Pharmacists can also help identify food triggers as a patient slowly works toward reintroducing foods. Moreover, dietary lactose restriction may be implemented, which can be beneficial for some patients with active IBD. Pharmacists should be mindful when a patient complains of symptoms suggestive of lactose intolerance (e.g., bloating, abdominal pain, and/or diarrhea); these patients should undergo a lactose hydrogen breath test to confirm a diagnosis of lactose intolerance, as it is thought that lactase gene downregulation may have a role in IBD. Lastly, some patients may supplement with EN even though it is not a routine component of induction therapy in adults with active IBD. However, EN may be given as an adjunct to induction therapy to patients with active disease who have undernutrition. Similarly, PN is usually not recommended, as the American Gastroenterological Association reviewed trials, including those in patients with active IBD, and concluded that, when given as induction therapy, PN provided no benefit over placebo.12,13
Patients in Remission: Which foods to eat and which to avoid is an important topic to discuss with patients with IBD in remission. In a guideline published in May 2020, a working group of the International Organization for the Study of IBD (IOIBD) provided updated expert consensus recommendations based on the best available evidence.5 This is much needed for IBD patients and practitioners, as this type of review on nutrition and diet for IBD has not been conducted before. Overall, the guidance emphasizes that although some patients associate certain types and/or quantities of food with development of abdominal discomfort and bloating, evidence does not link a particular food group to an increased risk of disease flare; therefore, it is not advised for most patients with IBD in remission to restrict any specific food group. However, there are key recommendations for both UC and CD that could aid patients with IBD in remission, and pharmacists can share these with their patients (TABLE 4).5 Evidence is insufficient to recommend restriction of wheat and gluten or any specific changes in consumption of complex carbohydrates or refined sugars and fructose.5
Apart from this recent IOIBD guidance, several nutritional and dietary interventions have been studied for maintaining remission in patients with IBD, but the benefits of these are unclear and outcomes may be inconclusive. These interventions are discussed in detail below.
Fiber—Generally, it is not necessary for IBD patients in remission to restrict fiber consumption. The recommended amount of dietary fiber is 14 grams per 1,000 calories. However, patients who are in remission but have chronic stricturing disease (involving luminal narrowing and/or previous bowel obstruction) should adhere to a low-fiber diet (maximum of 5 g daily). Dietary fiber should not be restricted in most patients because it may play a role in maintaining remission and also has a beneficial effect on commensal gut bacteria. Upon metabolism, some dietary fiber will form short-chain fatty acids, which have been shown to stimulate water and sodium absorption in the colon and to promote mucosal healing.14
Supplemental EN—Supplemental nutrition as primary maintenance therapy is not usually recommended, and efficacy studies have either been inconclusive or yielded mixed results.5,6
Elimination diet—This intervention is employed when patients believe that a specific food or food group accounts for their disease-related symptoms. One trial compared the use of glucocorticoids versus an elimination diet in 78 patients with IBD who had achieved remission with an elemental diet. Patients were instructed to introduce one new food group daily and to avoid foods that they knew previously precipitated their symptoms. Relapse rates at 2 years were lower in the diet-treated group than in the steroid-treated group (62% vs. 79%, P = .048). In the setting of IBD, an elimination diet may involve removing one specific food from the diet for a period of time and observing whether symptoms resolve during that time. An elimination diet may be used to introduce one new food at a time in order to identify foods that precipitate symptoms. Being readily accessible, pharmacists are well positioned to work with patients over time to identify foods that may precipitate or worsen their disease and to avoid such foods. Pharmacists can also consider assessment, and—if needed—may select the best supplementation options for calcium, selenium, magnesium, copper, vitamin A, and folate, particularly in patients who present with symptoms of deficiency and may have to avoid certain foods.14
Probiotics—Probiotics are living, nonpathogenic microorganisms that, when ingested, are believed to have the potential to exert a positive influence on host health and physiology. In patients with UC, some probiotics (e.g., Escherichia coli Nissle 1917, VSL #3) show promise for maintenance of remission, but no preparations have been validated for clinical use. In patients with CD, available data do not support the clinical effectiveness of probiotic therapy for either induction or maintenance of remission.5,6
Prebiotics—Prebiotics are nondigestible, selectively fermented carbohydrates that are thought to stimulate the growth and/or activity of a limited number of gut microbiota. To help patients understand the difference between probiotics and prebiotics, pharmacists can explain that probiotic foods and supplements are what one consumes to help support one’s microbiome and prebiotics are the support one gives the probiotics to help them do their job. In theory, this could help increase the good bacteria in the GI tract; however, there are data showing that it makes no difference. In a trial involving 103 patients with active CD who were assigned to fructo-oligosaccharides or placebo for 4 weeks, there was no significant difference in disease activity between the two groups.6
Low-carbohydrate diet—There have been anecdotal reports of a low-carbohydrate diet aiding in preventing relapse in patients with IBD. No major medical society has a recommendation supporting this diet. The Specific Carbohydrate Diet (SCD) is a highly restrictive low-carbohydrate diet that has been promoted for multiple chronic and autoimmune diseases. The diet is built on the premise that intestinal microbes that contribute to the development of IBD use carbohydrates as their primary energy source, leading to the production of acids and toxins that can injure the small intestine and further impair carbohydrate digestion and absorption. The SCD is free of grains, lactose, and sucrose. It also limits the intake of some legumes and prohibits ingestion of processed foods (due to additives in processed products). Unprocessed meats, poultry, fish, eggs, honey, noncanned vegetables, some legumes, fruits, nuts, homemade yogurt, and some lower-lactose cheeses are permitted. It is important to inform patients that data on SCD use in adults are limited. Although a case report showed improvement in SCD patients, many patients find the diet difficult to follow because of its restrictive nature. In addition, some clinicians express concern that it could lead to nutritional deficiencies. Randomized trials are required before the SCD can be recommended, but it is good for pharmacists to be aware of its parameters, as some patients may be following a low-carbohydrate diet.15
Antioxidants—Data regarding the use of antioxidants for patients with IBD are not substantial enough to make a recommendation for or against them. However, antioxidants neutralize oxygen free radicals (metabolic products that are increased during inflammatory states and result in significant tissue damage). Research has shown that patients with IBD have lower circulating levels of total antioxidants in the blood, and those with active disease have even lower amounts. The oxidative stress of the inflammation and the depletion of antioxidants are thought to contribute to damage to the intestinal walls. Therefore, in general, following an anti-inflammatory diet that is rich in antioxidants (e.g., omega-3 essential fatty acids, fruits, vegetables) may assist in reducing inflammation, and proinflammatory diets have been associated with increased risk of CD.16
Fish oils—Existing data do not support the use of fish oils for maintenance of remission in UC or CD. Two large placebo-controlled trials in CD patients and systematic reviews of clinical trials in patients with UC and CD found that oral ingested fish oil supplementation, while safe, is ineffective for inducing or maintaining remission in UC or CD. Despite these results, omega-3 polyunsaturated fatty acids—which are potent immunomodulatory substances—may reduce the production of inflammatory cytokines, and recommending their uptake from the diet would be prudent, especially for UC.5
Future Research
Finally, it is important to keep in mind that IBD is a complex disease. To fully understand the role of food intake in IBD, it is critical to further explore environmental factors (not just diet, but the use of pesticides and preservatives and the quality and type of soil the food is grown in), food processing (e.g., frying vs. boiling or baking), and potential bioactive food components that can induce intestinal inflammation and increase susceptibility to IBD.
Conclusion
Managing patients with a multifaceted condition such as IBD requires a multidisciplinary approach. This complex disease, with limited or inconclusive data on diet interventions, calls for a collaboration between the physician, dietitian, and pharmacist. Optimizing nutrition should become part of the strategic quality-care measures for IBD patients. For general dietary advice, pharmacists can counsel on the consensus guidance that advises patients with IBD to consume a diet composed of carbohydrates, fats, and protein and to limit intake of processed foods and artificial sweeteners while avoiding trans-fatty acids. Overall dietary advice for patients with IBD could emphasize a diet that comprises all sources of calories and is rich in fruits and vegetables and omega-3 fatty acids, with an emphasis on selecting and consuming in-season varieties that are freshly prepared without additives and preservatives.
REFERENCES
1. Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ³18 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166-1169.2. Nguyen GC, Chong CA, Chong RY. National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis. 2014;8(4):288-295.
3. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481-517.4. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 suppl A:5A-36A.
5. Levine A, Rhodes JM, Lindsay JO, et al. Dietary guidance from the International Organization for the Study of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(6):1381-1392.6. Bischoff SC, Escher J, Hébuterne X, et al. ESPEN practical guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2020;39(3):632-653.
7. Rollins C. Functional and meal replacement foods. In: Krinsky DL, Berardi RR, Ferreri SP, et al, eds. Handbook of Nonprescription Drugs. 17th ed. Washington, DC: American Pharmacists Association; 2012.8. National Cancer Institute. NCI Dictionary of Cancer Terms. Polymeric enteral nutrition formula. www.cancer.gov/dictionary?cdrid=658777. Accessed November 10, 2020.
9. Lochs H, Allison SP, Meier R, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: terminology, definitions and general topics. Clin Nutr. 2006;25(2):180-186.10. Nguyen GC, Laveist TA, Brant SR. The utilization of parenteral nutrition during the in-patient management of inflammatory bowel disease in the United States: a national survey. Aliment Pharmacol Ther. 2007;26(11-12):1499-1507.
11. Nguyen DL, Parekh N, Bechtold ML, Jamal MM. National trends and in-hospital outcomes of adult patients with inflammatory bowel disease receiving parenteral nutrition support. JPEN J Parenter Enteral Nutr. 2016;40(3):412-416.12. Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):707-717.
13. Eadala P, Matthews SB, Waud JP, et al. Association of lactose sensitivity with IBD—demonstrated by analysis of genetic polymorphism, breath gases and symptoms. Aliment Pharmacol Ther. 2011;34(7):735-746.14. Nazarenkov N, Seeger K, Beeken L, et al. Implementing dietary modifications and assessing nutritional adequacy of diets for inflammatory bowel disease. Gastroenterol Hepatol. 2019;15(3):133-144.
15. Nieves R, Jackson RT. Specific carbohydrate diet in treatment of inflammatory bowel disease. Tenn Med. 2004;97(9):407.16. Lo CH, Lochhead P, Khalili H, et al. Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2020;159(3):873-883.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





