US
Pharm. 2006;2:HS-28-HS-36.
Digoxin is a compound in the
drug family known as cardiac glycosides or cardenolides. The cardenolides
contain a five- or a six-membered lactone ring that is attached to a steroid
nucleus at position 17. Of the more than 300 known digitalis compounds, two
natural products have been used most often in clinical settings: ouabain and
digoxin. Ouabain is derived from the plant Strophanthus gratus, and
digoxin originates from the leaves of the purple foxglove (Digitalis
purpurea).1,2 Although the clinical efficacy of foxglove plant
extracts is a discovery attributed to English physician William Withering in
1785, these compounds have been used medicinally for more than 2,000 years.
3,4 Both a botanist and a physician, Withering knew of an herbal remedy
used for dropsy, a condition involving excess fluid retention. Dr. Withering
believed that digitalis produced a diuretic effect in those with an irregular,
weak pulse and concomitant edema.2,5
Current Indications and
Uses
Therapeutic
indications and uses for digoxin are based on its mechanisms of action, which
include effects on cardiac rate and rhythm, as well as effects on the force of
cardiac contraction. The slowing of rate and rhythm are attributed to
digoxin's impact on the central nervous system, leading to increased vagal
activity that results in slowed conduction in the atrioventricular (AV) node.
The increase in force of contraction is attributed to digoxin's binding to the
Na+/K+-ATPase pump. By binding to the K+
-binding site of the pump, digoxin leads to inhibition of the pump. The
consequent rise in Na+ concentration causes slowing of Ca++
efflux via the Na+/Ca++ exchanger and a relative
increase in intracellular Ca++. The extra Ca++ causes
the action potential of cardiac cells to be greater with more activation of
the contractile machinery.6
Control of ventricular rate
in the setting of atrial fibrillation has long been accomplished with digoxin.
In fact, for more than 200 years, digoxin was the main agent used for this
indication.7 Clinical trials have shown that 54% to 70% of patients
with atrial fibrillation are treated with digoxin.8,9 However,
digoxin is not effective in controlling ventricular rate in atrial
fibrillation during exercise.10 For this reason, some guidelines
recommend digoxin as second-line therapy for rate control in atrial
fibrillation.11 Still, digoxin is commonly used in this setting.
Recent data have raised the question of digoxin's potential to increase the
risk of stroke in patients with atrial fibrillation due to a possible role in
thrombogenesis mediated through increased intracellular calcium levels.12
This possibility could have substantial clinical implications because of the
large numbers of such patients receiving digoxin therapy. More trials are
needed to further test this theory.
Digoxin is an appealing
therapeutic option in elderly patients with atrial fibrillation. Unlike other
rate-control strategies (e.g., calcium channel blockers and beta-blockers),
digoxin does not cause hypotension or have negative inotropic effects.
However, caution is advised regarding potential drug interactions with digoxin
use in the elderly. In a clinical trial evaluating elderly patients admitted
to the hospital with specific drug toxicities, those on digoxin who
experienced toxicity were about 12 times as likely to have been treated with
clarithromycin in the previous week.13 Otherwise, provided that the
dosage is adjusted for renal function in elderly patients, digoxin can be an
inexpensive, well-tolerated therapy.14
Treatment of heart failure
is another historical use. Digoxin has been proven beneficial for symptomatic
control in sinus rhythm in patients with mild to moderate heart failure. In
the PROVED trial, symptoms that improved secondary to digoxin therapy included
ejection fraction, heart rate, and exercise capacity.15 In the
RADIANCE study, digoxin withdrawal resulted in clinical deterioration, such as
reductions in systolic function and worsening of exercise tolerance.16
However, no studies to date have shown any improvement in the incidence of
mortality with the use of digoxin in patients with heart failure.4
The most recent practice guidelines for the treatment of heart failure
recommend considering the addition of digoxin in patients with persistent
symptoms during therapy with diuretics, an angiotensin-converting enzyme (ACE)
inhibitor (or angiotensin receptor blocker), and a beta-blocker. Furthermore,
digoxin may be added to the initial regimen in patients with severe symptoms
who have not yet responded symptomatically during treatment with diuretics, an
ACE inhibitor, or a beta-blocker.17
Based on the results of the
Digitalis Investigation Group (DIG), digoxin is most often currently used for
its ability to reduce hospitalizations for declining heart failure.18
Evaluation of the DIG trial resulted in a revision of the current perspective
regarding therapeutic digoxin plasma concentrations.19,20 While
initially it appeared that digoxin may exhibit differing effects in men and
women, further analysis demonstrated the variations were more likely related
to differences in serum concentrations of digoxin.20,21 Digoxin
serum concentrations greater than 1.2 ng/mL lead to an increased risk of
mortality in patients with heart failure. Thus, the therapeutic range of
digoxin concentration currently recommended for the treatment of heart failure
is 0.5 to 0.9 ng/mL.19,20
Although digoxin was
historically used in the treatment of heart failure for its positive inotropic
effects, it has now become apparent that the neurohormonal effects of digoxin
may be equally or more important.22-25 Digoxin's effects on the
autonomic nervous system improve autonomic dysfunction in heart failure, as
indicated by decreases in plasma norepinephrine levels of up to 42%.26
Furthermore, digoxin has been shown to benefit outcomes in patients with
heart failure, even when patients remain in sinus rhythm, suggesting that the
beneficial effects are unrelated to the treatment of arrhythmia.27
Digoxin Pharmacokinetics
Digoxin
bioavailability varies based on the dosage. In tablet form, the
bioavailability ranges from 0.5 (50%) to more than 0.9 (90%); a value of 0.7
(70%) is often used as a standard for digoxin tablets.28 While
soft-gelatin digoxin capsules seem to be completely absorbed (bioavailability
=1.0), digoxin elixir exhibits a bioavailability of approximately 80% (0.8).
28 Administered intravenously, digoxin is assumed to have a
bioavailability of 100% (1.0). Such products as clarithromycin, erythromycin,
and itraconazole may increase digoxin's bioavailability, whereas charcoal,
cholestyramine, and St. John's wort may decrease it.
On average, the volume of
digoxin distribution is about 7.3 L/kg, based on ideal body weight.28
Thus, digoxin is distributed widely throughout the body. Although digoxin is
virtually insoluble in water, Na+/K+-ATPase pumps are
found in all tissues, and digoxin binds to these pumps, accounting for its
wide distribution throughout the body's tissues.29 This
characteristic is important in the treatment of digoxin toxicity with
digoxin-specific antibody fragments, as drug distributed in the tissue
compartments will reequilibrate following initial antibody fragment treatment.
Equations are also available
for more patient-specific calculations of digoxin's volume of distribution
that consider patient weight and creatinine clearance.28 In
addition, other factors may alter its volume of distribution: quinidine and
hypothyroidism decrease volume, and hyperthyroidism increases volume.28
Digoxin distributes relatively slowly, following a two-compartment model.
Complete distribution generally takes at least three to four hours. Since the
heart responds as part of the second compartment, therapeutic effects are
delayed until distribution is complete.
The clearance of digoxin
involves both metabolic and renal clearance from the body. In about 10% to 30%
of the population, metabolic elimination partially occurs as a result of
digoxin conversion by Eubacterium lentum in the gut to digoxin-reduction
products.30 Another component of digoxin metabolism is postulated
to occur because of hepatic conversion to 3-keto-digoxigenin and
3-epidigoxigenin metabolites, followed by conjugation.31
Additionally, digoxin is metabolized in the stomach by gastric acid, which
removes digitoxose sugars to form deglycosylated congeners. These sugars are
hydrolyzed, and the resulting products are oxidized and undergo epimerization
through hepatic uridine diphosphoglucose-glucuronosyltransferase, followed by
conjugation.32,33 Overall, the metabolic clearance of digoxin
averages approximately 0.8 mL/kg/minute.
Renal clearance of digoxin
is generally equivalent to creatinine clearance. In patients with heart
failure, both the metabolic and renal components of digoxin clearance
decrease, but the metabolic component decreases more dramatically. Clearance
of digoxin is also decreased in patients with hypothyroidism and in drug
interactions with amiodarone, quinidine, and verapamil. Alternatively,
clinical hyperthyroidism may increase digoxin clearance.28
In patients with normal
renal function, the half-life of digoxin ranges from 36 to 48 hours. In those
with renal insufficiency, the half-life can increase to six days.28,31
This has obvious implications for the timing of serum sampling for
measurement of serum digoxin levels, as discussed further in the following
section.
Measurement of Digoxin
Serum Concentrations
Considering there
is some overlap between therapeutic and toxic serum digoxin levels, symptoms
of toxicity may be reported in patients whose levels are within the
therapeutic range, while others may have no symptoms when their serum digoxin
levels are above the therapeutic threshold.31 As previously
mentioned, the therapeutic range for digoxin may be lower for patients with
heart failure than what is traditionally accepted (0.5 to 2 ng/mL).
19,20,28,31 However, digoxin's effects on rate control in atrial
fibrillation may require levels on the higher end of that range.31
Therefore, measurement of serum digoxin concentrations is necessary when
monitoring this medication to ensure its safe and effective use.
As is true in therapy with
any drug whose dosage is based on serum drug concentrations, routine
measurement of digoxin levels should occur once the steady state has been
reached. Since steady state is assumed following three to five half-lives of a
consistent dosing regimen, using five half-lives should ensure steady state
for a drug such as digoxin, which can demonstrate variations in
pharmacokinetic values, based on distribution and clearance. Specifically, in
a patient with normal renal function who receives digoxin therapy, steady
state should be achieved after at least seven to 10 days of treatment. In
patients experiencing end-stage renal disease, the lengthened half-life of
digoxin will translate into achievement of steady state, requiring 15 to 20
days.
Digoxin levels should be
measured once steady state has occurred, but the distribution of a given dose
must also be taken into consideration. Due to the relatively long distribution
phase of digoxin, drawing levels within this phase can be avoided best by
drawing trough levels. However, if one must draw a level sooner for practical
timing concerns, waiting at least four hours after an intravenous dose or six
hours after an oral dose is generally sufficient.28
Circumstances that
necessitate the measurement of digoxin serum concentrations are the subject of
some debate. Some recommended indications for the cost-effective use of serum
digoxin monitoring include measurement: (1) following initial digoxin doses;
(2) to ascertain patient adherence with therapy; (3) in patients with dynamic
or impaired renal function; (4) in patients receiving potentially interacting
concomitant medications; (5) in patients not experiencing adequate clinical
response; and (6) to prevent and diagnose toxicity.34 If
measurement is limited to these situations and performed following the
guidelines related to achieving steady state and digoxin distribution,
clinically useful levels can be reliably attained.
Some individuals, including
neonates, pregnant women, patients with renal failure, and those with hepatic
failure, who are not taking digoxin possess digoxin-like immunoreactive
substances that can interfere with the measurement of digoxin levels via
immunoassay.35,36 Awareness of this occurrence can ensure that
clinicians heed such factors when interpreting serum digoxin concentrations. A
patient's clinical condition should always be considered in conjunction with
measured serum concentrations when adjusting digoxin-dosing regimens so that
serum concentrations are not the sole indicator used in the decision-making
process.
Digoxin Drug Interactions
Digoxin is known
to interact with a wide variety of medications (table 1). One mechanism of
drug interaction with digoxin is change in absorption due to increased contact
time in the small intestine. This can occur with concomitant use of
anticholinergic agents, e.g., atropine, diphenhydramine, phenothiazines,
scopolamine, and benztropine, which slow gastrointestinal motility.37
Two other mechanisms believed to account for many drug interactions with
digoxin are the inhibition of P-glycoprotein, located in the brush borders of
the proximal tubule, and inhibition of digoxin metabolism, secondary to a lack
of Eubacterium lentum in the gastrointestinal tract.30 The
antibiotics clarithromycin, erythromycin, and tetracycline alter the flora of
the gut, leading to decreased digoxin metabolism and consequent increases in
digoxin levels.30,37 The antiarrhythmics quinidine, amiodarone, and
verapamil inhibit P-glycoprotein in the kidney, resulting in decreased renal
clearance of digoxin.37
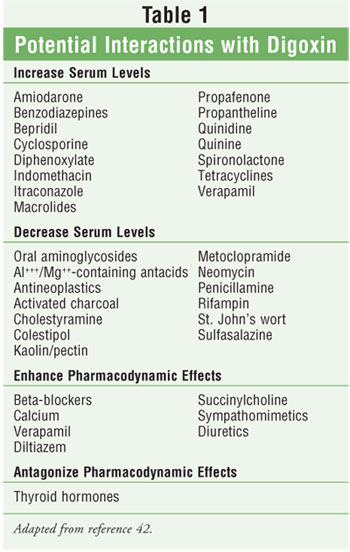
Digoxin can lead to
life-threatening hyperkalemia. This potential adverse effect of digoxin could
cause interactions with medications that also affect potassium homeostasis,
such as ACE inhibitors, angiotensin receptor–blocking drugs, spironolactone,
eplerenone, and potassium supplements.37 Both pharmacokinetic and
pharmacodynamic mechanisms should be noted regarding digoxin drug interactions.
Risk Factors
Patients at
highest risk for digoxin toxicity include those with renal insufficiency,
heart failure, and dehydration.37 Hypoxia secondary to chronic
pulmonary disease, hypokalemia, hypomagnesemia, and hypercalcemia are also
indicated to increase the risk of developing arrhythmias induced by digoxin.
2,38 The mechanism for the increase in digoxin toxicity risk secondary
to hypokalemia derives from the fact that when K+ is low, more K
+-binding sites are open for digoxin binding, increasing the effective
concentration of digoxin within the heart.6
Signs and Symptoms
Although digoxin
toxicity may lead to the development of any type of arrhythmia, bradycardia
and AV block are predicted conditions due to digoxin's mechanism of action.
The inhibition of the Na+/K+ pump by digoxin leads to an
increase in intracellular Ca++. This increase in Ca++
then leads to an increase in the strength of contraction or inotropy. However,
these same pharmacological effects that cause inotropy may also cause the
development of arrhythmias.39 In the event of severe intoxication,
such as that seen in suicide attempts, both severe hyperkalemia and extreme
bradycardia occur.2 The hyperkalemia is a result of digoxin
inhibition of the Na+/K+-ATPase activity in skeletal
muscle.2,40
When digoxin levels in the
body are elevated, adverse effects due to accumulation in the central nervous
system may occur. Some of these effects include blurred vision, xanthopsia
(disturbances in color vision), and retrobulbar optic neuritis.2,38
Additional effects that may be seen because of mediation of the central
nervous system by digoxin include nausea, vomiting, increased respiration
rate, excitation, headache, malaise, drowsiness, dizziness, and apathy.
4,38 Notably, cardiac symptoms of toxicity may appear before noncardiac
symptoms.38
Treatment of Digoxin Toxicity
Activated
charcoal can be used in the treatment of digoxin toxicity. The use of
activated charcoal can lead to a 30% to 40% drop in digoxin levels within 12
to 18 hours. Unlike the use of digoxin antibodies, the drop in digoxin levels
produced by activated charcoal avoids complete reversal of the therapeutic
effects of digoxin in patients using the medication for treatment of cardiac
disease.41 This may be a beneficial strategy in patients whose
digoxin concentrations do not greatly exceed those in the therapeutic range
and who could benefit from conservative medical care. Additionally, supportive
care involving potassium administration, discontinuation of digoxin therapy,
and assessment of magnesium and calcium levels should be employed as indicated
by the patient's clinical condition.41
Digoxin-specific antibody
fragments, or digoxin immune Fab, was introduced in the 1970s and is indicated
for the treatment of life-threatening or potentially life-threatening digoxin
toxicity or overdose.40,42 The two products currently available in
the U.S. market are Digibind and DigiFab. Both of these products are ovine in
origin, collected and purified from sheep immunized with human albumin
conjugated with digoxin. Digoxin molecules bind preferentially to the antibody
fragments, making them unavailable for binding to their receptors. The
digoxin-antibody complexes are then renally eliminated.
The clinical conditions
indicating the need for these products as defined in their package inserts
include the following: acute ingestion of greater than 10 mg of digoxin in
adults or 4 mg of digoxin in children, acute ingestion of digoxin leading to a
serum level of more than 10 ng/mL, chronic ingestion of digoxin leading to a
serum level higher than 6 ng/mL in adults or 4 ng/mL in children, or
manifestations of life-threatening digoxin toxicity, such as severe
ventricular arrhythmias, progressive bradycardia, second- or third-degree
heart block not responsive to atropine, or serum potassium levels exceeding 5
mEq/L in adults or 6 mEq/L in children with rapidly progressive signs and
symptoms of digoxin toxicity.42 Digibind has also been suggested
and used in the treatment of poisoning with oleander, bufadienolide-containing
aphrodisiacs, digitoxin, and foxglove extract.4
For both brands of digoxin
immune Fab, one vial of the product will bind approximately 0.5 mg of digoxin.
Therefore, the dose of digoxin immune Fab is based on the amount of excess
digoxin believed to be present in the patient experiencing toxicity. In some
cases, this amount is known, such as in situations of suicide attempt with
deliberate overdose or unintentional ingestion by a child. However, in cases
of chronic ingestion, this may be more difficult to ascertain, especially as
the toxicity may have developed over time with changes in renal function. To
calculate digoxin immune Fab dose for patients experiencing an acute ingestion
of digoxin, one must first determine the total body load of digoxin. This can
be accomplished by multiplying the amount of digoxin ingested (in milligrams)
by the bioavailability for the product ingested (0.7 for tablets). To
determine the total body load of digoxin (in milligrams) for patients
experiencing toxicity as a result of chronic ingestion of digoxin, one should
multiply the serum digoxin level (in ng/mL) by the volume of distribution of
digoxin (7.3 L/kg) by the patient's ideal body weight (in kg) and divide by
1,000. Once the body load of digoxin is determined, the amount should be
divided by 0.5, to account for the approximate amount of digoxin neutralized
by one vial of digoxin immune Fab, to determine the number of vials of digoxin
immune Fab that should be administered.
An understanding of both
digoxin and digoxin-immune Fab pharmacokinetics is crucial to developing a
therapeutic dosing regimen.40 The volume of distribution for
digoxin immune Fab is approximately 0.35 L/kg, indicating penetration into the
extracellular space.42 However, this volume is much smaller than
that of digoxin, signifying that shifts from deeper tissue stores of digoxin
may occur as the antibody complexes with digoxin in the central circulation as
well as more accessible tissue stores.40 The half-life of digoxin
immune Fab is reported to be between 15 and 30 hours.40,42 This
pharmacokinetic parameter is important from the standpoint that if the entire
dose of digoxin immune Fab is given at one time, it may be eliminated from the
body before digoxin reequilibration from deeper tissue stores and an optimal
degree of digoxin-antibody complexing can occur. For this reason, it has been
recommended that half of the calculated necessary digoxin immune Fab dose be
given initially, in both acute and chronic poisoning situations, followed by
additional doses administered in one to two hours if no clinical response is
seen or in six to 12 hours if toxicity recurs.40
The costs associated with
digoxin toxicity should be considered. It has been shown that the mean overall
cost associated with digoxin toxicity is approximately $4,000 per episode.
43 This cost may be somewhat variable with the use of digoxin immune
Fab, especially in the treatment of patients with renal dysfunction and a
serum digoxin concentration of 2.3 ng/mL or higher. In such cases, the
use of digoxin immune Fab can result in a reduction in length of stay and
overall lower treatment costs.44
Because papain is used in
the process of producing digoxin immune Fab, patients with hypersensitivity to
papain, chymopapain, other papaya extracts, or the pineapple enzyme bromelain
may be at risk for such a reaction. Additionally, patients with allergies to
latex or dust mites may have cross-sensitivity to papain and experience
hypersensitivity to digoxin immune Fab. Finally, those with allergies to sheep
or ovine products or who have previously received ovine products may be at
increased risk for hypersensitivity to digoxin immune Fab. The benefit of
using this product in such patients should be weighed against the risks, and
as a safety measure, treatment for anaphylaxis should be readily available.
42
Summary
Digoxin toxicity
can occur as a result of many situations, including drug interactions,
electrolyte abnormalities, changes in renal function, acute ingestion of large
amounts of the substance, or chronic ingestion of doses larger than necessary
for therapeutic effects. Clinicians should monitor patients for the signs and
symptoms of digoxin toxicity while utilizing preventive measures. Such
preventive measures should include appropriate digoxin serum concentration
measurement, evaluation of pharmacotherapy regimens for potential drug
interactions, assessment of electrolytes, and digoxin regimen determination
based on pharmacokinetic parameters. If digoxin toxicity occurs, treatment
should be implemented based on the patient's clinical condition. With
appropriate care, digoxin can be an efficacious, safe, and cost-effective
treatment.
REFERENCES
1. Smith TW,
Haber E. Digitalis (first of four parts). N Engl J Med.
1973;289:945-952.
2. Rocco TP, Fang JC,
Roden DM. Cardiac glycosides. In: Brunton LL, Lazo JS, Parker KL, eds.
Goodman & Gilman's The Pharmacological Basis of Therapeutics.
11th ed. New York: McGraw-Hill; 2006:886-889, 921-923.
3. Withering W. An
Account of the Foxglove and Some of its Medical Uses: With Practical Remarks
on Dropsy and Other Diseases. London: J and J Robinson; 1785.
4. Jortani SA, Valdes
R Jr. Digoxin and its related endogenous factors. Crit Rev Clin Lab Sci
. 1997;34:225-274.
5. Eichhorn EJ,
Gheorghiade M. Digoxin--new perspective on an old drug. N Engl J Med
. 2002;347:1394-1395.
6. Rang HP, Dale MM,
Ritter JM, Gardner P. Pharmacology. New York: Churchill Livingstone;
1995:283-284.
7. Khan IA, Nair CK,
Singh N, et al. Acute ventricular rate control in atrial fibrillation and
atrial flutter. Int J Cardiol. 2004;97:7-13.
8. Van Gelder IC,
Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control
in patients with recurrent persistent atrial fibrillation. N Engl J Med
. 2002;347:1834-1840.
9. Wyse DG, Waldo AL,
DiMarco JP, et al. A comparison of rate control and rhythm control in patients
with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
10. Beasley R, Smith
DA, McHaffie DJ. Exercise heart rates at different serum digoxin
concentrations in patients with atrial fibrillation. Br Med J.
1985;290:9-11.
11. Holten KB. How
should we manage newly diagnosed atrial fibrillation? J Fam Prac.
2004;53:641-643.
12. Chirinos JA,
Castrellon A, Zambrano JP, et al. Digoxin use is associated with increased
platelet and endothelial cell activation in patients with nonvalvular atrial
fibrillation. Heart Rhythm. 2005;2:525-529.
13. Juurlink DN,
Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients
hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
14. Rosenfeld LE.
Atrial fibrillation: how to approach rate control. Curr Cardiol Rep.
2005;7:391-397.
15. Uretsky BF, Young
JB, Shahidi FE, et al. Randomized study assessing the effect of digoxin
withdrawal in patients with mild to moderate chronic congestive heart failure:
results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol
. 1993;22:955-962.
16. Packer M,
Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with
chronic heart failure treated with angiotensin-converting-enzyme inhibitors.
RADIANCE Study. N Engl J Med. 1993;329:1-7.
17. ACC/AHA 2005
Guideline Update for the Diagnosis and Management of Chronic Heart Failure in
the Adult: A Report of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines (Writing Committee to Update the
2001 Guidelines for the Evaluation and Management of Heart Failure). J Am
Coll Cardiol. 2005;46:1-82.
18. Garg R, Gorlin R,
Smith T, Yusuf S, on behalf of the Digitalis Investigation Group. The effect
of digoxin on mortality and morbidity in patients with heart failure. N
Engl J Med. 1997;336:525-533.
19. Adams KF Jr,
Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to
mortality and morbidity in women in the Digitalis Investigation Group Trial.
J Am Coll Cardiol. 2005;46:497-504.
20. Rathore SS,
Curtis JP, Wang Y, et al. Association of serum digoxin concentration and
outcomes in patients with heart failure. JAMA. 2003;289:871-878.
21. Rathore SS, Wang
Y, Krumholz HM. Sex-based differences in the effect of digoxin for the
treatment of heart failure. N Engl J Med. 2002;347:1403-1411.
22. Terra SG, Washam
JB, Dunham GD, Gattis WA. Therapeutic range of digoxin's efficacy in heart
failure: what is the evidence? Pharmacotherapy. 1999;19:1123-1126.
23. Slatton ML, Irani
WN, Hall SA, et al. Does digoxin provide additional hemodynamic and autonomic
benefit at higher doses in patients with mild to moderate heart failure and
normal sinus rhythm? J Am Coll Cardiol. 1997;29:1206-1213.
24. Packer M. The
neurohormonal hypothesis: a theory to explain the mechanism of disease
progression in heart failure. J Am Coll Cardiol. 1992;20:248-254.
25. Newton GE, Tong
JH, Schofield AM, et al. Digoxin reduces cardiac sympathetic activity in
severe congestive heart failure. J Am Coll Cardiol. 1996;28:155-161.
26. Krum H, Bigger T,
Goldsmith RL, et al. Effect of long-term digoxin therapy on autonomic function
in patients with chronic heart failure. J Am Coll Cardiol.
1995;25:289-294.
27. The Task Force of
the Working Group on Heart Failure of the European Society of Cardiology. The
treatment of heart failure. Eur Heart J. 1997;18:736-753.
28. Winter ME.
Digoxin. In: Winter ME. Basic Clinical Pharmacokinetics. 4th ed.
Baltimore: Lippincott Williams & Wilkins; 2004:183-221.
29. Clausen T. The
Na+, K+ pump in skeletal muscle: quantification, regulation and functional
significance. Acta Physiol Scand. 1996;156:227-235.
30. Hirata S, Izumi
S, Furukubo T, et al. Interactions between clarithromycin and digoxin in
patients with end-stage renal disease. Int J Clin Pharmacol Ther.
2005;43:30-36.
31. Mutnick AH.
Digoxin. In: Schumacher GE, ed. Therapeutic Drug Monitoring. Norwalk:
Appleton & Lange; 1995:469-491.
32. Gault MH, Charles
JD, Sugden DI, et al. Hydrolysis of digoxin by acid. J Pharm Pharmacol.
1980;29:27-32.
33. Gault MH, Karla
J, Ahmed M, et al. Influence of gastric pH on digoxin biotransformation. I.
Intragastric hydrolysis. Clin Pharmacol Ther. 1980;27:16-21.
34. Lewis RP.
Clinical use of serum digoxin concentrations. Am J Cardiol.
1992;69:97G-107G.
35. Pudek MR,
Seccombe DW, Jacobson BE, Humphries K. Effect of assay conditions on cross
reactivity of digoxin-like immunoreactive substance(s) with radioimmunoassay
kits. Clin Chem. 1985;31:1806-1810.
36. Way BA, Wilhite
TR, Miller R, et al. Vitros digoxin immunoassay evaluated for interference by
digoxin-like immunoreactive factors. Clin Chem. 1998;44:1339-1340.
37. Prybys KM. Deadly
drug interactions in emergency medicine. Emerg Med Clin North Am.
2004;22:845-863.
38. Parker RB,
Patterson JH, Johnson JA. Heart failure. In: DiPiro JT, Talbert RL, Yee GC, et
al., eds. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New
York: McGraw-Hill; 2005:219-260.
39. Rocchetti M,
Besana A, Mostacciuolo G, et al. Diverse toxicity associated with cardiac
Na+/K+ pump inhibition: evaluation of electrophysiological mechanisms. J
Pharmacol Exp Ther. 2003;305:765-771.
40. Bateman DN.
Digoxin-specific antibody fragments: how much and when? Toxicol Rev.
2004;23:135-143.
41. Fee WH Jr.
Activated charcoal safe and effective for digoxin toxicity [letter]. Am J
Med. 2004;116:430.
42. Facts &
Comparisons 4.0. Digoxin immune Fab. Available at:
online.factsandcomparisons.com. Accessed December 12, 2005.
43. Gandhi AJ,
Vlasses PH, Morton DJ, Bauman JL. Economic impact of digoxin toxicity.
Pharmacoeconomics. 1997;12:175-181.
44. DiDomenico RJ,
Walton SM, Sanoski CA, Bauman JL. Analysis of the use of digoxin immune fab
for the treatment of non-life-threatening digoxin toxicity. J Cardiovasc
Pharmacol Ther. 2000;5:77-85.
To comment on this article,
contact editor@uspharmacist.com.






