US Pharm. 2016:41(1)HS9-HS12.
ABSTRACT: Alteplase (Activase), an intravenous (IV) tissue plasminogen activator (tPA), is the only FDA-approved thrombolytic agent for acute ischemic stroke (AIS). Recently updated American Heart Association/American Stroke Association (AHA/ASA) Guidelines for the Early Management of Patients with Acute Ischemic Stroke and revisions to Activase labeling have expanded the time window eligibility for tPA treatment and have loosened contraindications surrounding tPA treatment. These changes should promote increased utilization of tPA in AIS, resulting in more instances of timely reperfusion and reduction of neurologic sequelae.
Stroke affects 795,000 people annually and is the fifth leading cause of death in the United States, resulting in 129,000 deaths each year.1 Stroke is a leading cause of disability and is associated with $33.6 billion in health service costs, medication use, and loss of productivity.1 Stroke morbidity can be reduced by emergent medical treatment that minimizes brain injury and decreases disability and death. Although there are two types of stroke, ischemic and hemorrhagic, this review will focus specifically on the treatment of acute ischemic stroke (AIS).
Importance of Timely Reperfusion in AIS
During AIS, brain tissue is deprived of oxygen, usually resulting in two regions of ischemia, the core and the penumbra. The core, where the blood clot occurs, receives roughly 10% to 25% of normal blood flow, leading to infarction and tissue necrosis.2 The penumbra, which is the ischemic tissue surrounding the core, can receive blood from collateral circulation, delaying completion of the infarct. These neurons are salvageable if reperfused in time.2 IV administration of alteplase (a recombinant tissue plasminogen activator [tPA], thrombolytic agent, and the only FDA-approved pharmacologic treatment for AIS), can lyse the offending clot, restore blood flow, prevent further neuronal loss, and possibly even completely reverse deficits if given early enough.3 For this reason, “time is brain” during AIS.4 Nearly 2 million neurons are lost during each minute of untreated stroke, and each hour during this time period is comparable to 3.6 years of normal aging.4 Thus, shorter door-to-needle times with regard to IV tPA administration can save more brain cells and vastly improve functional outcomes. Clinical studies support this, as IV tPA treatment within 1.5 hours of symptom onset has been shown to have the best statistically significant outcome, followed by treatment within 1.5 to 3 hours and then treatment within 3 to 4.5 hours.3 Based on this, The Joint Commission (TJC) and the American Heart Association/American Stroke Association (AHA/ASA) further advocate a door-to-needle time of 60 minutes for IV tPA administration once the patient arrives at the hospital.3,5
While there is clear data to support its timely use, IV tPA remains significantly underutilized. Data collected from the Get with the Guidelines Stroke program showed that only 7% of all stroke patients arrived within a time period eligible for IV tPA, and only 77% of patients eligible for tPA were given tPA within 2 hours of symptom onset.6 Efforts to increase utilization and timeliness of IV tPA delivery have included increasing public awareness of stroke and teaching individuals to seek immediate medical attention. F.A.S.T. is an easy way to have patients recognize common signs of stroke: Face drooping, Arm weakness, Speech difficulty, and Time to call 9-1-1.7 Calling 9-1-1 and utilizing emergency medical services (EMS) is especially emphasized in AIS, as this decreases prehospital delays, reduces time to physician evaluation and brain imaging, mobilizes the stroke team, and most importantly, enhances tPA use. 8 Updates to the AHA/ASA guidelines for the early management of AIS published in 2013 and revisions to tPA labeling introduced in February 2015 should also promote increased IV tPA use and are reviewed below.3,9
Expansion of Time Window for IV tPA Eligibility in AIS
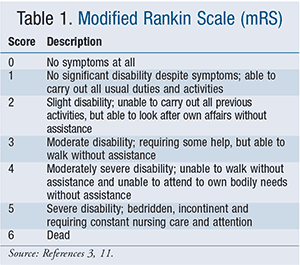
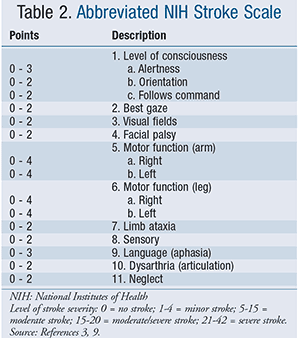
Alteplase was FDA approved under the brand name Activase in 1997.3 Its introduction radically changed AIS care, as stroke became one of the few true neurologic emergencies having timely treatment that could change outcomes. The drug’s approval was largely based on results of the National Institute of Neurological Disorders and Stroke (NINDS) trial, which showed improved neurologic outcomes at 3 months after stroke when IV tPA was given within 3 hours of symptom onset, with best outcomes in those treated within 90 minutes.10 While FDA labeling still indicates that patients eligible for IV tPA should be treated within 3 hours, the 2013 AHA/ASA treatment guidelines for AIS recommend that IV tPA also be administered to eligible patients 3 to 4.5 hours after stroke, largely based on results of the European Cooperative Acute Stroke Study III (ECASS III) trial, which concluded that tPA administered within this time period still significantly improved clinical outcomes in patients with AIS (specifically, modified Rankin scores [TABLE 1] on the global disability scale at 90 days).3,11 The ECASS III trial, however, excluded patients who were >80 years of age, taking oral anticoagulants regardless of international normalized ratio (INR), had more severe strokes (e.g., baseline National Institutes of Health Stroke Scale (NIHSS) score of >25; TABLE 2), had especially large strokes (e.g., imaging evidence of ischemic injury involving more than one-third of the middle cerebral artery territory), and had a history of both stroke and diabetes.11 Therefore, patients are eligible for IV tPA if they present within the 3 to 4.5 hours of symptom onset, but the 2013 AHA/ASA treatment guidelines list the above as additional exclusion criteria.
Updated Labeling of IV tPA in AIS
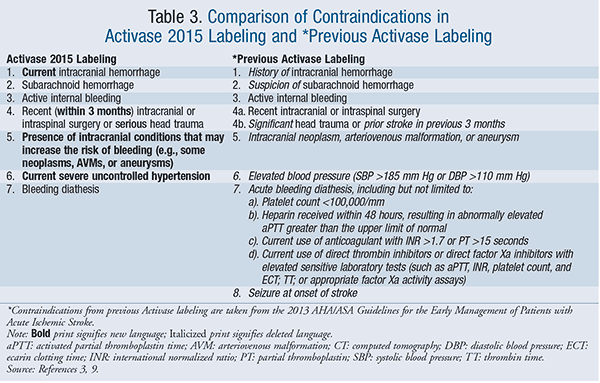
Unfortunately, IV tPA significantly increases the risk of severe, potentially fatal intracranial hemorrhage (ICH).10,11 It is recognized that careful patient selection is the best way to prevent the occurrence of intracranial hemorrhage and optimize the benefit-to-risk ratio of IV tPA.10 Thus, a host of contraindications, based on the exclusion criteria used in the NINDS and ECASS-III trials, have accompanied the use of IV tPA in AIS since its approval. However, physicians with expertise in stroke care, supported by guideline statements stating such, have often modified this list of contraindications, as it is increasingly recognized that IV tPA can still provide benefit with minimal risk in many patients, even when contraindications do exist.3 Updates to the FDA labeling of Activase, introduced in February 2015, better reflect this current clinical practice. Whereas prior labeling indicated evaluating the use of Activase against the consequence of “significant disability or death,” new Activase labeling generally reduces the limitations of use and allows for greater clinical judgment of risk versus benefit.9
In the updated labeling, history of ICH is no longer a contraindication, although current hemorrhage is still appropriately contraindicated, as is subarachnoid hemorrhage, active internal bleeding, serious head trauma, and recent intracranial or intraspinal surgery (“recent” is more clearly defined as being within 3 months). Additionally, the presence of intracranial neoplasms, arteriovenous malformations, or aneurysms are no longer considered absolute contraindications; if present, the clinician is allowed to judge the potential bleeding propensity of these lesions when deciding whether or not to give tPA. Furthermore, blood pressure and bleeding diathesis parameters (e.g., platelet count, activated partial thromboplastin time [aPTT], INR, etc.) are no longer defined, again allowing the provider more room to use clinical judgment when weighing the risk versus benefit of tPA. Furthermore, the new labeling completely eliminates contraindications regarding prior strokes and seizure at onset of stroke. These changes are reviewed side-by-side in TABLE 3.9
Current Use of IV tPA in AIS
Currently, IV tPA should be considered for all patients presenting with AIS having symptom onset of less than 4.5 hours prior.3 However, as emphasized above, all efforts should be made to achieve a door-to-needle time of 60 minutes or less as recommended by Joint Commission and AHA/ASA.3 It should also be noted that while labeling for IV tPA has changed, updated guidelines reflecting these labeling changes have not yet been published. Time-eligible patients should be assessed for tPA use based on individual patient characteristics, taking into consideration the 2013 AHA/ASA stroke treatment guidelines and updated contraindications. Specifically, assessment of intracranial conditions that may increase risk of bleeding such as size, location, and bleeding propensity; assessment of severe uncontrolled hypertension, such as risk of pressure-related ICH; and assessment of acute bleeding diatheses, such as platelet abnormalities, elevated aPTT, elevated INR, or possibility of recent (within 24 hours) use of direct factor Xa inhibitors should still be performed by a physician with expertise in stroke to determine the benefit-to-risk ratio of tPA use in individual patients. 3,9 As stroke guidelines also list blood glucose <50 mg/dL and computed tomography (CT) demonstrating multilobar infarction encompassing greater than one-third of the cerebral hemisphere as contraindications, blood glucose should also be assessed for individual patients and corrected as clinically appropriate, and infarct size should be evaluated for its propensity to bleed. For those patients who present within the 3 to 4.5 hour window, additional assessment regarding age, use of oral anticoagulants, NIHSS score, and a history of both stroke and diabetes should be performed with appropriate clinical judgment made on suitability of tPA therapy.
Dosing of tPA
For patients in whom tPA is deemed appropriate, the recommended tPA dose for AIS is 0.9 mg/kg (maximum dose 90 mg) infused intravenously over 60 minutes with 10% of the total dose administered as an initial bolus over 1 minute.9 In contrast to the above discussion concerning contraindications, there are currently no on-label or off-label indications for deviations from this dosing, and deviations are not recommended.
Role of Other IV Thrombolytic Agents in AIS
Of the available thrombolytic agents, IV use of tenecteplase and streptokinase have been most extensively studied in AIS. Although initial studies suggested greater reperfusion with fewer bleeding complications, a phase 2b study of tenecteplase in AIS was prematurely terminated as a result of slow enrollment and inability to determine a “best” dose; its role in AIS remains inconclusive.12,13 Some have suggested that it may have a role in those with confirmed allergy to alteplase, although this has yet to be determined. Clinical studies involving streptokinase in AIS have shown high rates of hemorrhage and increased mortality; its use is not recomended.14,15
Role of Endovascular Therapy in AIS
IV tPA remains the mainstay of early treatment in AIS, and all patients eligible for IV tPA should receive this treatment. Selected patients, depending on the location of the causative occlusion and other factors, may also be eligible for endovascular therapy with stent retrievers if presenting within 6 hours of symptom onset. It is important for pharmacists to be aware that unlike treatment of acute myocardial infarctions in which the patient receives IV thrombolytic therapy or a more invasive endovascular procedure such as percutaneous transluminal coronary angioplasty (PTCA), AIS patients who are eligible for endovascular therapy should receive both IV thrombolytic therapy and the more invasive endovascular procedure using stent retrievers. It is also important for pharmacists to know that endovascular therapy requires the patient to be at an experienced stroke center with rapid access to qualified neurointerventionalists and advanced imaging technologies, including cerebral angiography. Lastly, pharmacists should be aware that regional systems of stroke care are being developed and consist of primary stroke centers that provide initial emergency care, including administration of IV tPA, and comprehensive stroke centers capable of performing endovascular stroke treatment with comprehensive periprocedural care.16
TJC Core Measures and Stroke
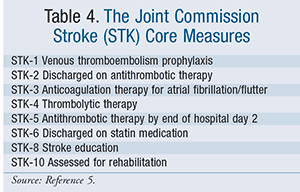
Because of the wide impact of stroke, The Joint Commission (TJC) has identified it as a focus area for core measures of hospital quality of care. In an effort to promote appropriate use of IV tPA, a key stroke core measure is whether or not eligible patients received IV thrombolytic therapy. If the patient does not receive IV tPA, TJC further requires documentation in the medical chart of the reason why this was not done. Seven other important stroke core measures, though of a less acute nature than tPA administration, have also been identified; all the measures are listed in TABLE 4.5
Conclusion
Timely restoration of blood flow in AIS is effective in reducing long-term morbidity. Quick identification of stroke warning signs and rapid initiation of emergency medical services will promote early assessment of IV tPA eligibility, the main treatment modality for AIS. Pharmacists should be aware that patient and provider education concerning techniques for quick identification of stroke warning signs (e.g., F.A.S.T.); rapid activation of emergency medical services (e.g., call 9-1-1); and appropriate patient selection for IV tPA therapy, including knowledge of the expanded time window for tPA eligibility and revised labeling regarding tPA contraindications, will aid in early identification, assessment, and administration of IV tPA in AIS. Furthermore, awareness of systems of care surrounding neuroendovascular therapies will promote the use of this additional treatment modality and further enhance timely stroke care.
REFERENCES
1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-322.
2. Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3(1):47-55.
3. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
4. Saver JL. Time is brain—quantified. Stroke. 2006;37(1):263-266.
5. Joint Commission Website. Specifications manual for national hospital inpatient quality measures. www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. Accessed July 8, 2015.
6. Schwamm LH, Fonarow GC, Reeves MJ, et al. Get with the guidelines–stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107-115.
7. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
8. Acker JE, Pancioli AM, Crocco TJ, et al. Implementation strategies for emergency medical services within stroke systems of care: a policy statement from the American Heart Association/American Stroke Association Expert Panel on Emergency Medical Services Systems and the Stroke Council. Stroke. 2007;38(11):3097-3115.
9. Activase [package insert]. South San Francisco, CA: Genentech, Inc; February 2015.
10. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581-1587.
11. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
12. Haley EC, Thompson JL, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010;41(4):707-711.
13. Huang X, Cheripelli BK, Lloyd SM, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015;14(4):368-376.
14. Thrombolytic therapy with streptokinase in acute ischemic stroke. The Multicenter Acute Stroke Trial—Europe Study Group. N Engl J Med. 1996;335(3):145-150.
15. Hommel M, Boissel JP, Cornu C, et al. Termination of trial of streptokinase in severe acute ischaemic stroke. MAST Study Group. Lancet. 1995;345(8941):57.
16. Powers WJ, Derdeyn CP, Biller J, et al. 2015 AHA/ASA Focused Update of the 2013 Guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020-3035.
To comment on this article, contact rdavidson@uspharmacist.com.